Abstract
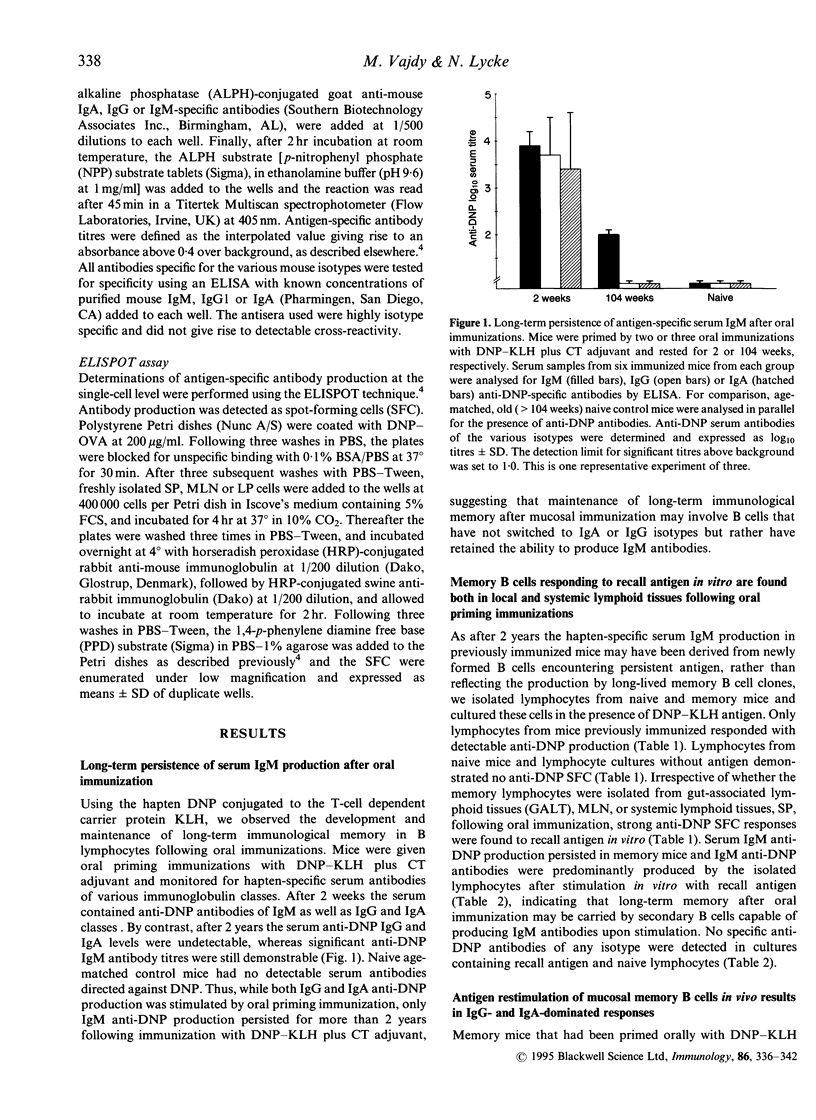
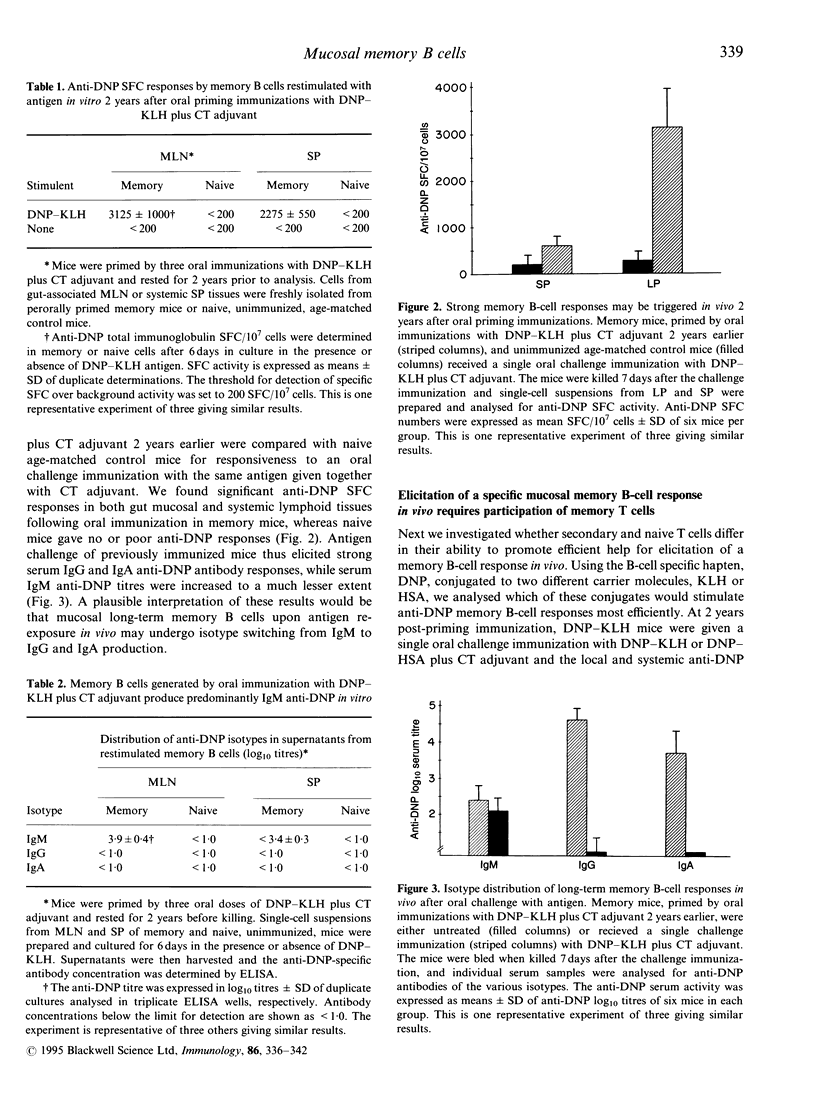
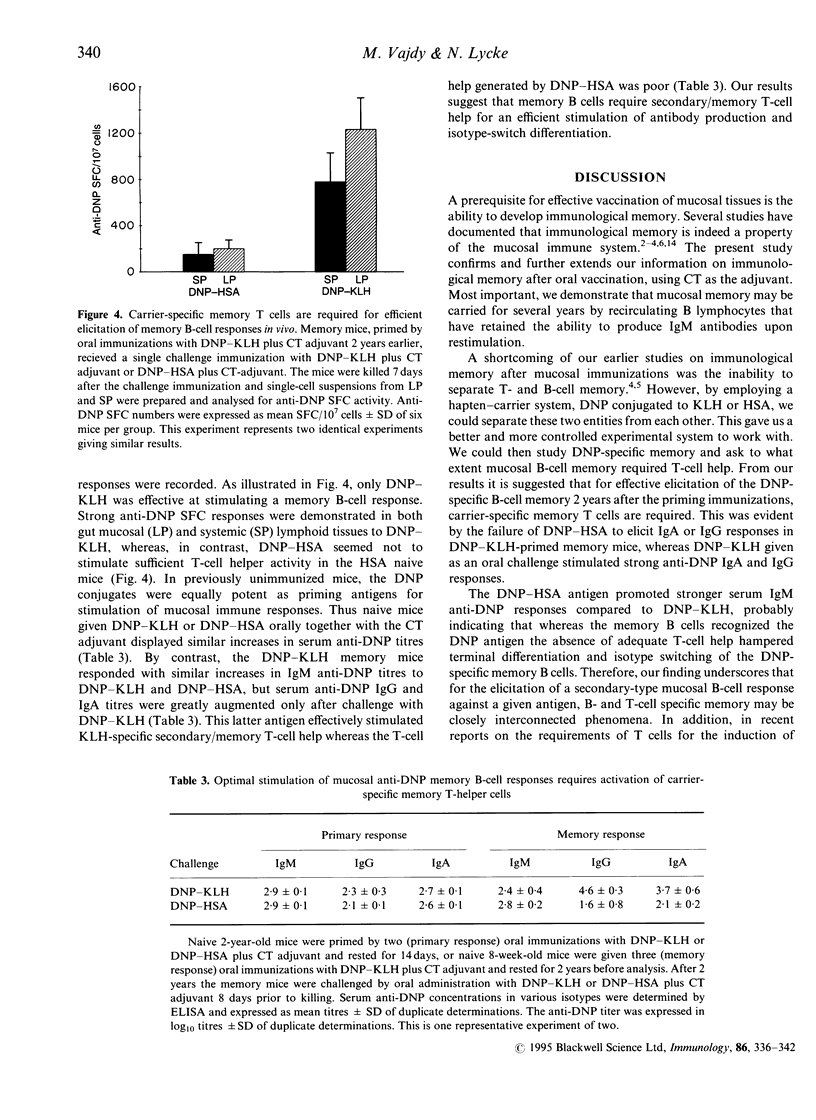
In recent studies we have demonstrated that immunological B- and T-cell memory may be stimulated effectively by oral immunization, simply by admixing protein antigens with cholera toxin (CT) adjuvant. Here we extend information by employing a hapten-carrier system allowing us to separate B- and T-cell memory and to evaluate the requirement of memory T cells for effective reactivation of mucosal memory B cells. We found that 2 weeks following oral priming immunizations with dinitrophenyl-keyhole limpet haemocyanin (DNP-KLH) plus CT adjuvant, significant serum anti-DNP antibodies of IgG, IgA and IgM immunoglobulin classes were demonstrated. However, after 2 years only IgM anti-DNP antibodies could still be detected in serum. When memory lymphocytes were isolated from these mice, from both systemic and gut-associated lymphoid tissues, and challenged with antigen in vitro, vigorous IgM, but no IgG or IgA, anti-DNP production was observed. By contrast, when the DNP-KHL-primed memory mice were challenged in vivo by an oral booster immunization with DNP-KLH plus CT adjuvant, strong systemic IgG and local mucosal IgA anti-DNP responses were recorded, while IgM anti-DNP production was poor. Moreover, the mucosal memory B cells from DNP-KHL-immunized mice were more responsive in vivo to an oral booster immunization with the carrier-specific antigen, DNP-KLH, compared to that provided by an unrelated carrier, DNP-human serum albumin (HSA), which gave only poor mucosal and systemic anti-DNP B-cell responses. Taken together our data suggest that mucosal memory B cells are recirculating cells that have retained their ability to produce IgM antibodies and, therefore, have not undergone switch differentiation involving gene rearrangements with constant mu-chain deletions. Furthermore, mucosal B-cell memory and CD4+ T-cell memory are closely interconnected phenomena, requiring both components for effective expression and probably also for maintenance of immunological memory in the mucosal immune system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann M. F., Kündig T. M., Odermatt B., Hengartner H., Zinkernagel R. M. Free recirculation of memory B cells versus antigen-dependent differentiation to antibody-forming cells. J Immunol. 1994 Oct 15;153(8):3386–3397. [PubMed] [Google Scholar]
- Bäckström M., Lebens M., Schödel F., Holmgren J. Insertion of a HIV-1-neutralizing epitope in a surface-exposed internal region of the cholera toxin B-subunit. Gene. 1994 Nov 18;149(2):211–217. doi: 10.1016/0378-1119(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Colle J. H., Truffa-Bachi P., Freitas A. A. Secondary antibody responses to thymus-independent antigens. Decline and life-span of memory. Eur J Immunol. 1988 Sep;18(9):1307–1314. doi: 10.1002/eji.1830180902. [DOI] [PubMed] [Google Scholar]
- Ermak T. H., Owen R. L. Phenotype and distribution of T lymphocytes in Peyer's patches of athymic mice. Histochemistry. 1987;87(4):321–325. doi: 10.1007/BF00492585. [DOI] [PubMed] [Google Scholar]
- Foy T. M., Laman J. D., Ledbetter J. A., Aruffo A., Claassen E., Noelle R. J. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994 Jul 1;180(1):157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Dullforce P., Jainandunsing S. Memory B cell development but not germinal center formation is impaired by in vivo blockade of CD40-CD40 ligand interaction. J Exp Med. 1994 Jul 1;180(1):141–155. doi: 10.1084/jem.180.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991 Nov 1;174(5):969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988 Nov 3;336(6194):70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Ishii R., Yamasaki K., Kishimoto T., Hardy R. R. Isolation of high-affinity memory B cells: phycoerythrin as a probe for antigen-binding cells. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1379–1383. doi: 10.1073/pnas.84.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Kataoka T. Organization of immunoglobulin heavy chain genes and allelic deletion model. Proc Natl Acad Sci U S A. 1978 May;75(5):2140–2144. doi: 10.1073/pnas.75.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenz D., Teale J. M., Klinman N. R., Strober S. Surface IgG-bearing cells retain the capacity to secrete IgM. J Immunol. 1986 Mar 15;136(6):2076–2079. [PubMed] [Google Scholar]
- Lagergard T., Shiloach J., Robbins J. B., Schneerson R. Synthesis and immunological properties of conjugates composed of group B streptococcus type III capsular polysaccharide covalently bound to tetanus toxoid. Infect Immun. 1990 Mar;58(3):687–694. doi: 10.1128/iai.58.3.687-694.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebman D. A., Griffin P. M., Cebra J. J. Relationship between expression of IgA by Peyer's patch cells and functional IgA memory cells. J Exp Med. 1987 Nov 1;166(5):1405–1418. doi: 10.1084/jem.166.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. C., Shockett P., Stavnezer J. Regulation of the antibody class switch to IgA. Immunol Res. 1991;10(3-4):376–380. doi: 10.1007/BF02919724. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol. 1986 May;23(5):611–616. doi: 10.1111/j.1365-3083.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Dertzbaugh M. T., Eldridge J. H., Hirasawa M., Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10(2):75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- Milligan G. N., Fairchild R. L., Sterner K. E., Braley-Mullen H. Type 6 and 19 pneumococcal polysaccharides coupled to erythrocytes elicit pneumococcal cell wall-specific primary IgM responses and capsular polysaccharide-specific secondary IgG responses. Eur J Immunol. 1990 Mar;20(3):595–603. doi: 10.1002/eji.1830200320. [DOI] [PubMed] [Google Scholar]
- Perlmutter A. P., Gilbert W. Antibodies of the secondary response can be expressed without switch recombination in normal mouse B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7189–7193. doi: 10.1073/pnas.81.22.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J. Control of lymphocyte homing. Curr Opin Immunol. 1994 Jun;6(3):394–406. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw B. R., Fanslow W. C., 3rd, Armitage R. J., Campbell K. A., Liggitt D., Wright B., Davison B. L., Maliszewski C. R. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994 Nov 1;180(5):1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman P., Chen Y. Y., Lutzker S., Li S. C., Stewart V., Coffman R., Alt F. W. Structure and expression of germ line immunoglobulin heavy-chain epsilon transcripts: interleukin-4 plus lipopolysaccharide-directed switching to C epsilon. Mol Cell Biol. 1990 Apr;10(4):1672–1679. doi: 10.1128/mcb.10.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittek B., Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990 Aug 23;346(6286):749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. E., Burgess A. W. Retention of intact HSA for prolonged periods in the popliteal lymph nodes of specifically immunized mice. Cell Immunol. 1979 Jun;45(1):207–212. doi: 10.1016/0008-8749(79)90378-2. [DOI] [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992 Mar;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Stimulation of antigen-specific T- and B-cell memory in local as well as systemic lymphoid tissues following oral immunization with cholera toxin adjuvant. Immunology. 1993 Oct;80(2):197–203. [PMC free article] [PubMed] [Google Scholar]
- Yaoita Y., Kumagai Y., Okumura K., Honjo T. Expression of lymphocyte surface IgE does not require switch recombination. Nature. 1982 Jun 24;297(5868):697–699. doi: 10.1038/297697a0. [DOI] [PubMed] [Google Scholar]


