Abstract
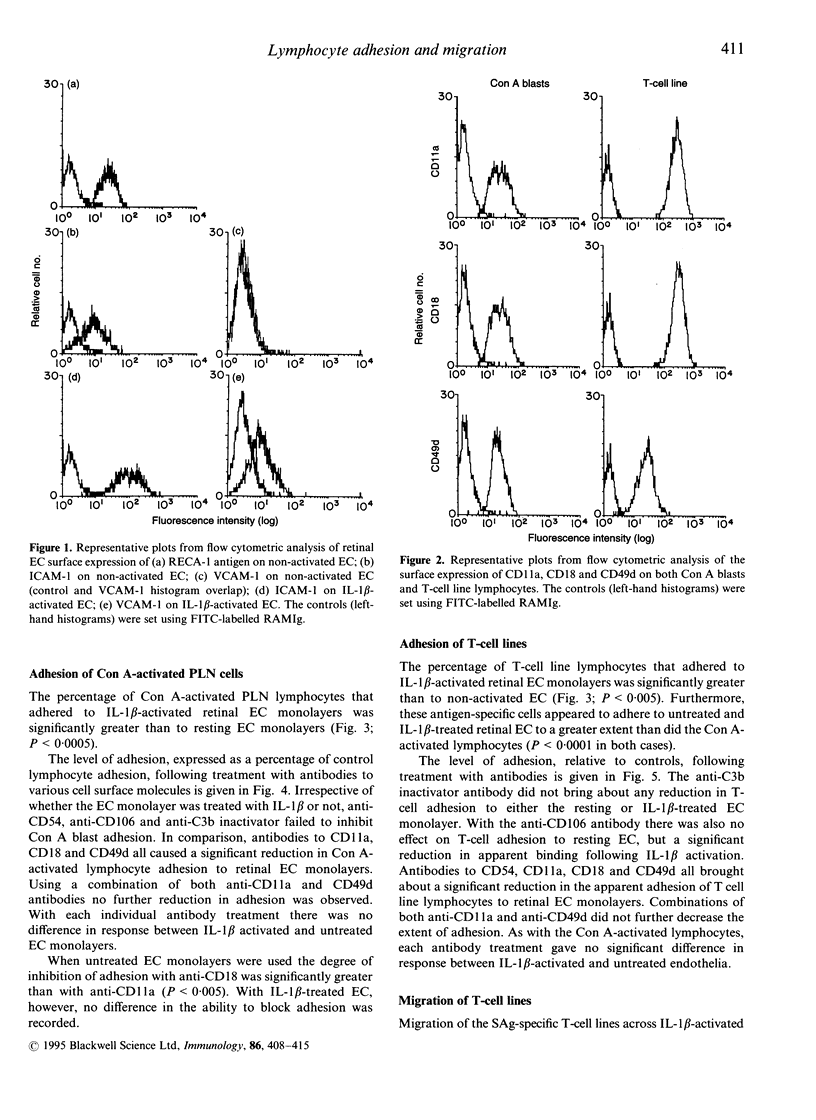
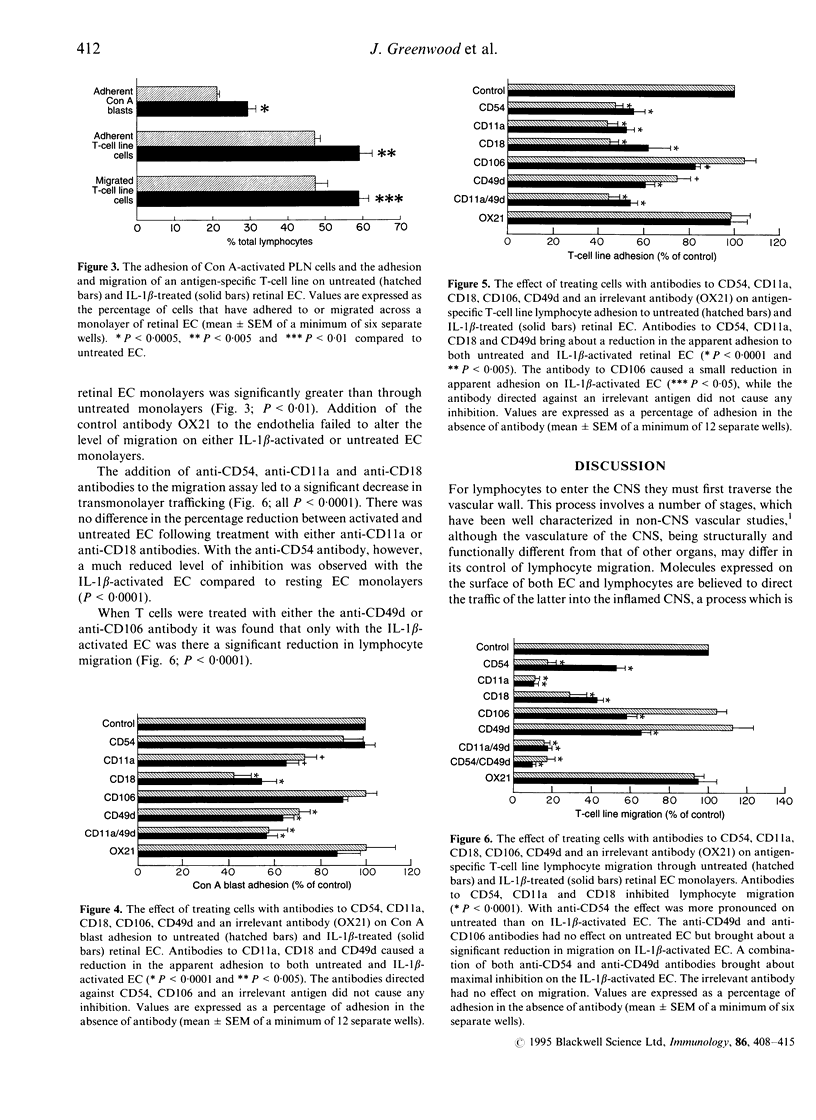
Lymphocyte adhesion to and migration across endothelial cell (EC) monolayers, derived from the rat blood-retinal barrier (BRB), were measured in vitro. The binding of concanavalin A (Con A)-activated peripheral lymph node lymphocytes and the migration of CD4+ T-cell lines could be significantly increased by treating the EC with interleukin-1 beta (IL-1 beta). To determine the role of various adhesion molecules during the processes of lymphocyte binding and transmonolayer migration (diapedesis), lymphocytes were treated with monoclonal antibody (mAb) specific for CD11a (alpha L subunit of leucocyte functional antigen-1; LFA-1), CD18 (beta 2 subunit of leucam family) and CD49d (alpha 4 subunit of very late activation antigen-4; VLA-4) and EC with mAb specific for CD54 (intercellular adhesion molecule-1; ICAM-1) and CD106 (vascular cell adhesion molecule-1; VCAM-1). Binding of the highly adhesive but non-migratory Con A-activated lymphocytes was inhibited by mAb to CD11a (reduced to 73% and 65% of control lymphocyte adhesion) and CD18 (42% and 54%) on non-activated and IL-1 beta-treated EC, respectively, but not by mAb to ICAM-1 or VCAM-1. Diapedesis of the highly migratory T-cell line lymphocytes was also blocked by antibodies to CD11a (reduced to 11% and 10% of control T-cell migration), CD18 (29% and 43%) but in addition was also inhibited by anti-ICAM-1 (17% and 53%) on non-activated and IL-1 beta treated EC, respectively. Both anti-VLA-4 and anti-VCAM-1 were also effective in producing a smaller reduction in migration, but only on IL-1 beta activated EC (66% and 58% of control migration, respectively). These studies indicate that lymphocyte adhesion to central nervous system (CNS) vascular EC is largely dependent on LFA-1 but not through its interaction with ICAM-1. In contrast, lymphocyte diapedesis is mostly supported through the LFA-1/ICAM-1 pairing, with a small proportion being mediated by VLA-4/VCAM-1 on IL-1 beta-activated EC. This latter pathway, however, also appears to be dependent on LFA-1 interacting with the EC.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron J. L., Madri J. A., Ruddle N. H., Hashim G., Janeway C. A., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993 Jan 1;177(1):57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt A. M., Jones H. C. Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res. 1992 Jan 8;569(1):100–105. doi: 10.1016/0006-8993(92)90374-i. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., van Goor H., Klatter F., Majoor G. D., van Bussel E., van Breda Vriesman P. J. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992 Apr;66(4):459–466. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Fabry Z., Waldschmidt M. M., Hendrickson D., Keiner J., Love-Homan L., Takei F., Hart M. N. Adhesion molecules on murine brain microvascular endothelial cells: expression and regulation of ICAM-1 and Lgp 55. J Neuroimmunol. 1992 Jan;36(1):1–11. doi: 10.1016/0165-5728(92)90026-h. [DOI] [PubMed] [Google Scholar]
- Greenwood J., Calder V. L. Lymphocyte migration through cultured endothelial cell monolayers derived from the blood-retinal barrier. Immunology. 1993 Nov;80(3):401–406. [PMC free article] [PubMed] [Google Scholar]
- Greenwood J. Characterization of a rat retinal endothelial cell culture and the expression of P-glycoprotein in brain and retinal endothelium in vitro. J Neuroimmunol. 1992 Jul;39(1-2):123–132. doi: 10.1016/0165-5728(92)90181-j. [DOI] [PubMed] [Google Scholar]
- Greenwood J., Howes R., Lightman S. The blood-retinal barrier in experimental autoimmune uveoretinitis. Leukocyte interactions and functional damage. Lab Invest. 1994 Jan;70(1):39–52. [PubMed] [Google Scholar]
- Hourihan H., Allen T. D., Ager A. Lymphocyte migration across high endothelium is associated with increases in alpha 4 beta 1 integrin (VLA-4) affinity. J Cell Sci. 1993 Apr;104(Pt 4):1049–1059. doi: 10.1242/jcs.104.4.1049. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Male D. K., Lantos P. L. Adhesion of lymphocytes to cerebral microvascular cells: effects of interferon-gamma, tumour necrosis factor and interleukin-1. Immunology. 1988 Aug;64(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B., Wykretowicz A. Effect of a new monoclonal antibody, TA-2, that inhibits lymphocyte adherence to cytokine stimulated endothelium in the rat. J Immunol. 1991 Jul 1;147(1):109–116. [PubMed] [Google Scholar]
- Kavanaugh A. F., Lightfoot E., Lipsky P. E., Oppenheimer-Marks N. Role of CD11/CD18 in adhesion and transendothelial migration of T cells. Analysis utilizing CD18-deficient T cell clones. J Immunol. 1991 Jun 15;146(12):4149–4156. [PubMed] [Google Scholar]
- Lossinsky A. S., Badmajew V., Robson J. A., Moretz R. C., Wisniewski H. M. Sites of egress of inflammatory cells and horseradish peroxidase transport across the blood-brain barrier in a murine model of chronic relapsing experimental allergic encephalomyelitis. Acta Neuropathol. 1989;78(4):359–371. doi: 10.1007/BF00688172. [DOI] [PubMed] [Google Scholar]
- Male D., Pryce G., Linke A., Rahman J. Lymphocyte migration into the CNS modelled in vitro. J Neuroimmunol. 1992 Oct;40(2-3):167–171. doi: 10.1016/0165-5728(92)90130-d. [DOI] [PubMed] [Google Scholar]
- Male D., Pyrce G., Hughes C., Lantos P. Lymphocyte migration into brain modelled in vitro: control by lymphocyte activation, cytokines, and antigen. Cell Immunol. 1990 Apr 15;127(1):1–11. doi: 10.1016/0008-8749(90)90109-5. [DOI] [PubMed] [Google Scholar]
- Male D., Rahman J., Pryce G., Tamatani T., Miyasaka M. Lymphocyte migration into the CNS modelled in vitro: roles of LFA-1, ICAM-1 and VLA-4. Immunology. 1994 Mar;81(3):366–372. [PMC free article] [PubMed] [Google Scholar]
- Martiney J. A., Berman J. W., Brosnan C. F. Chronic inflammatory effects of interleukin-1 on the blood-retina barrier. J Neuroimmunol. 1992 Dec;41(2):167–176. doi: 10.1016/0165-5728(92)90067-u. [DOI] [PubMed] [Google Scholar]
- McCarron R. M., Wang L., Racke M. K., McFarlin D. E., Spatz M. Cytokine-regulated adhesion between encephalitogenic T lymphocytes and cerebrovascular endothelial cells. J Neuroimmunol. 1993 Mar;43(1-2):23–30. doi: 10.1016/0165-5728(93)90071-6. [DOI] [PubMed] [Google Scholar]
- O'Neill J. K., Butter C., Baker D., Gschmeissner S. E., Kraal G., Butcher E. C., Turk J. L. Expression of vascular addressins and ICAM-1 by endothelial cells in the spinal cord during chronic relapsing experimental allergic encephalomyelitis in the Biozzi AB/H mouse. Immunology. 1991 Apr;72(4):520–525. [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991 Nov 1;147(9):2913–2921. [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Lipsky P. E. Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol. 1990 Jul 1;145(1):140–148. [PubMed] [Google Scholar]
- Pankonin G., Reipert B., Ager A. Interactions between interleukin-2-activated lymphocytes and vascular endothelium: binding to and migration across specialized and non-specialized endothelia. Immunology. 1992 Sep;77(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Pryce G., Santos W., Male D. An assay for the analysis of lymphocyte migration across cerebral endothelium in vitro. J Immunol Methods. 1994 Jan 3;167(1-2):55–63. doi: 10.1016/0022-1759(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Cannella B., Duijvestijn A. M., Cross A. H. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990 Oct;63(4):476–489. [PubMed] [Google Scholar]
- Sedgwick J. D., MacPhee I. A., Puklavec M. Isolation of encephalitogenic CD4+ T cell clones in the rat. Cloning methodology and interferon-gamma secretion. J Immunol Methods. 1989 Jul 26;121(2):185–196. doi: 10.1016/0022-1759(89)90159-2. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Gopal T. V., Horgan K. J., Graber N., Beall L. D., van Seventer G. A., Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991 Jun;113(5):1203–1212. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Miyasaka M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit-specific monoclonal antibodies. Eur J Immunol. 1991 Mar;21(3):627–633. doi: 10.1002/eji.1830210314. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2(2):165–171. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Potter J., Vachula M., Smith C. W., Anderson D. C. Suppression of human lymphocyte chemotaxis and transendothelial migration by anti-LFA-1 antibody. J Immunol. 1989 Nov 15;143(10):3207–3210. [PubMed] [Google Scholar]
- Vennegoor C. J., van de Wiel-van Kemenade E., Huijbens R. J., Sanchez-Madrid F., Melief C. J., Figdor C. G. Role of LFA-1 and VLA-4 in the adhesion of cloned normal and LFA-1 (CD11/CD18)-deficient T cells to cultured endothelial cells. Indication for a new adhesion pathway. J Immunol. 1992 Feb 15;148(4):1093–1101. [PubMed] [Google Scholar]
- Waldschmidt M. M., Fabry Z., Keiner J., Love-Homan L., Hart M. N. Adhesion of splenocytes to brain microvascular endothelium in the BALB/c and SJL/j mouse systems. J Neuroimmunol. 1991 Dec;35(1-3):191–200. doi: 10.1016/0165-5728(91)90173-5. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Calder V. L., Greenwood J., Lightman S. L. Lymphocyte adhesion to cultured endothelial cells of the blood-retinal barrier. J Neuroimmunol. 1993 Nov-Dec;48(2):161–168. doi: 10.1016/0165-5728(93)90188-5. [DOI] [PubMed] [Google Scholar]
- Wilcox C. E., Ward A. M., Evans A., Baker D., Rothlein R., Turk J. L. Endothelial cell expression of the intercellular adhesion molecule-1 (ICAM-1) in the central nervous system of guinea pigs during acute and chronic relapsing experimental allergic encephalomyelitis. J Neuroimmunol. 1990 Nov;30(1):43–51. doi: 10.1016/0165-5728(90)90051-n. [DOI] [PubMed] [Google Scholar]
- Wysocki J., Issekutz T. B. Effect of T cell activation on lymphocyte-endothelial cell adherence and the role of VLA-4 in the rat. Cell Immunol. 1992 Apr;140(2):420–431. doi: 10.1016/0008-8749(92)90208-7. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Zhao Z. S., Calder V. L., McLauchlan M., Lightman S. L. Differential lymphokine expression by rat antigen-specific CD4+ T cell lines with antigen and mitogen. Cell Immunol. 1994 Dec;159(2):220–234. doi: 10.1006/cimm.1994.1309. [DOI] [PubMed] [Google Scholar]
- de Vries H. E., Moor A. C., Blom-Roosemalen M. C., de Boer A. G., Breimer D. D., van Berkel T. J., Kuiper J. Lymphocyte adhesion to brain capillary endothelial cells in vitro. J Neuroimmunol. 1994 Jun;52(1):1–8. doi: 10.1016/0165-5728(94)90155-4. [DOI] [PubMed] [Google Scholar]


