Abstract
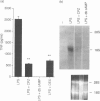
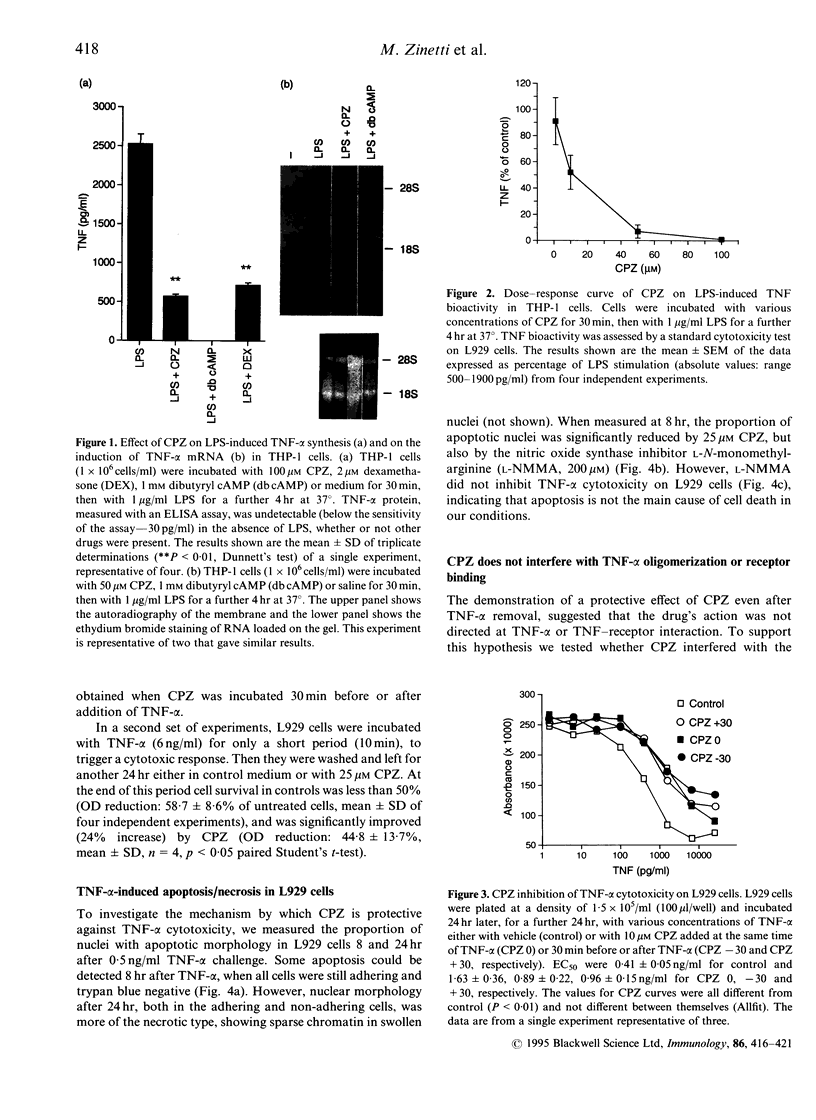
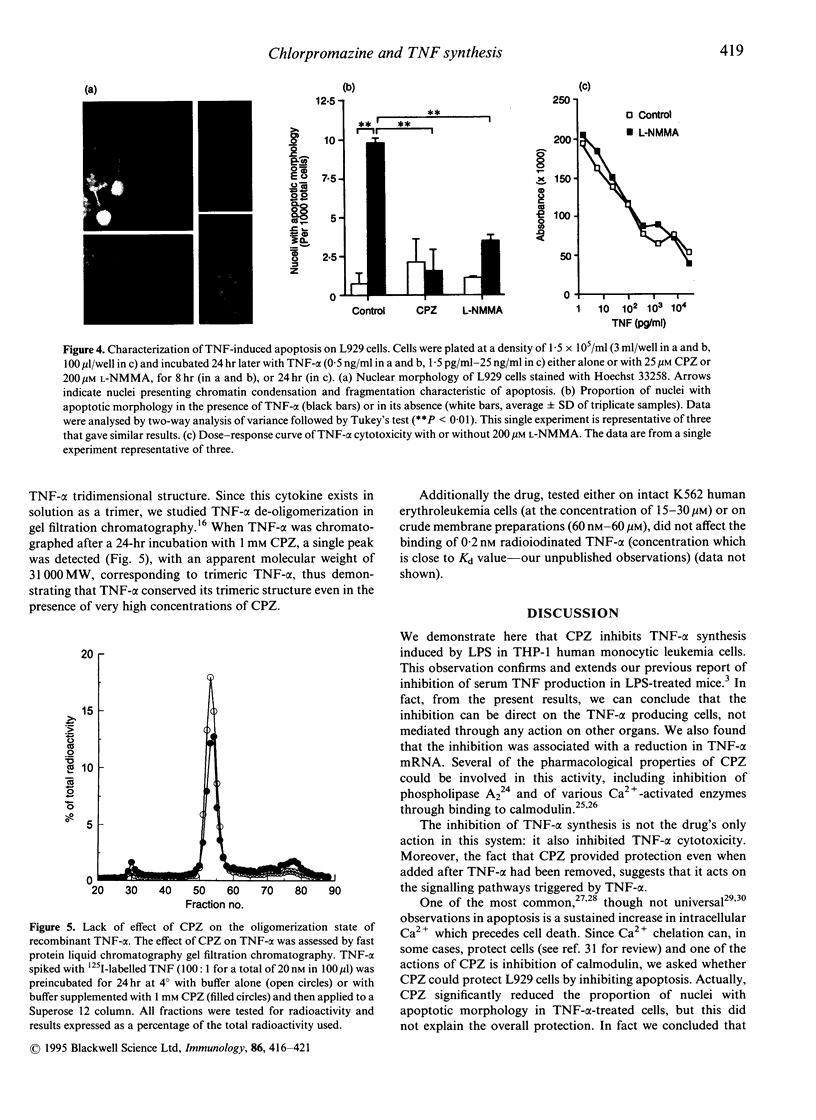
Chlorpromazine (CPZ) has been previously shown to protect against endotoxin [lipopolysaccharide (LPS)] lethality and inhibit the release of tumour necrosis factor in vivo. We investigated at the cellular level whether this was due to direct inhibition of tumour necrosis factor-alpha (TNF-alpha) synthesis, using LPS-stimulated THP-1 human monocytic leukemia cells. We also studied the effect of CPZ on human TNF-alpha action by assessing TNF-alpha cytotoxicity on mouse fibrosarcoma L929 cells. CPZ (1-100 microM) inhibited TNF-alpha production in THP-1 cells in a dose dependent manner by a maximum of 80%. This effect was comparable to that of two well-known inhibitory drugs, dexamethasone and cyclicAMP. Inhibition was also evident at the mRNA level. On the other hand CPZ (10-25 microM) also inhibited TNF-alpha activity: in fact it reduced the cytotoxicity of TNF-alpha on L929 cells (EC50 was increased four times) and could provide protection even as a post-treatment. CPZ inhibited TNF-induced apoptosis in L929 cells, as detected by analysis of nuclear morphology. However, since we showed that apoptosis was very limited, and was not the main mode of cell death in our conditions, this could not explain the overall protection. Since CPZ did not interfere with either the oligomerization state of TNF-alpha or its receptor binding, our data suggest that it reduced cytotoxicity by inhibiting some steps in the TNF-alpha signalling pathways.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Alnemri E. S., Litwack G. Activation of internucleosomal DNA cleavage in human CEM lymphocytes by glucocorticoid and novobiocin. Evidence for a non-Ca2(+)-requiring mechanism(s). J Biol Chem. 1990 Oct 5;265(28):17323–17333. [PubMed] [Google Scholar]
- Alzani R., Corti A., Grazioli L., Cozzi E., Ghezzi P., Marcucci F. Suramin induces deoligomerization of human tumor necrosis factor alpha. J Biol Chem. 1993 Jun 15;268(17):12526–12529. [PubMed] [Google Scholar]
- Bailly S., Ferrua B., Fay M., Gougerot-Pocidalo M. A. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990 May;2(3):205–210. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Eastman A. Endonuclease activation during apoptosis: the role of cytosolic Ca2+ and pH. Biochem Biophys Res Commun. 1992 Jul 31;186(2):782–789. doi: 10.1016/0006-291x(92)90814-2. [DOI] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Mengozzi M., Ghezzi P. Protective effect of chlorpromazine against the lethality of interleukin 1 in adrenalectomized or actinomycin D-sensitized mice. Biochem Biophys Res Commun. 1989 Dec 29;165(3):942–946. doi: 10.1016/0006-291x(89)92694-6. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Corti A., Fassina G., Marcucci F., Barbanti E., Cassani G. Oligomeric tumour necrosis factor alpha slowly converts into inactive forms at bioactive levels. Biochem J. 1992 Jun 15;284(Pt 3):905–910. doi: 10.1042/bj2840905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Endres S., Fülle H. J., Sinha B., Stoll D., Dinarello C. A., Gerzer R., Weber P. C. Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-alpha and interleukin-1 beta by human mononuclear cells. Immunology. 1991 Jan;72(1):56–60. [PMC free article] [PubMed] [Google Scholar]
- Gadina M., Bertini R., Mengozzi M., Zandalasini M., Mantovani A., Ghezzi P. Protective effect of chlorpromazine on endotoxin toxicity and TNF production in glucocorticoid-sensitive and glucocorticoid-resistant models of endotoxic shock. J Exp Med. 1991 Jun 1;173(6):1305–1310. doi: 10.1084/jem.173.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Fratelli M. Activation of apoptosis by serum deprivation in a teratocarcinoma cell line: inhibition by L-acetylcarnitine. Exp Cell Res. 1993 Jan;204(1):54–60. doi: 10.1006/excr.1993.1008. [DOI] [PubMed] [Google Scholar]
- Heller R. A., Krönke M. Tumor necrosis factor receptor-mediated signaling pathways. J Cell Biol. 1994 Jul;126(1):5–9. doi: 10.1083/jcb.126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jue D. M., Sherry B., Luedke C., Manogue K. R., Cerami A. Processing of newly synthesized cachectin/tumor necrosis factor in endotoxin-stimulated macrophages. Biochemistry. 1990 Sep 11;29(36):8371–8377. doi: 10.1021/bi00488a025. [DOI] [PubMed] [Google Scholar]
- Laster S. M., Wood J. G., Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988 Oct 15;141(8):2629–2634. [PubMed] [Google Scholar]
- Mason R. P., Chignell C. F. Free radicals in pharmacology and toxicology--selected topics. Pharmacol Rev. 1981 Dec;33(4):189–211. [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S. Ca2+ and cell death. Ann N Y Acad Sci. 1992 May 11;648:17–27. doi: 10.1111/j.1749-6632.1992.tb24520.x. [DOI] [PubMed] [Google Scholar]
- Rainteau D., Wolf C., Bereziat G., Polonovski J. Binding of a spin-labelled chlorpromazine analogue to calmodulin. Biochem J. 1984 Aug 1;221(3):659–663. doi: 10.1042/bj2210659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H., Gong J. H., Schmidt A., Nain M., Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct 1;141(7):2388–2393. [PubMed] [Google Scholar]
- Roufogalis B. D. Phenothiazine antagonism of calmodulin: a structurally-nonspecific interaction. Biochem Biophys Res Commun. 1981 Feb 12;98(3):607–613. doi: 10.1016/0006-291x(81)91157-8. [DOI] [PubMed] [Google Scholar]
- Testa R., Abbiati G., Ceserani R., Restelli G., Vanasia A., Barone D., Gobbi M., Mennini T. Profile of in vitro binding affinities of neuroleptics at different rat brain receptors: cluster analysis comparison with pharmacological and clinical profiles. Pharm Res. 1989 Jul;6(7):571–577. doi: 10.1023/a:1015997213587. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Treves S., Trentini P. L., Ascanelli M., Bucci G., Di Virgilio F. Apoptosis is dependent on intracellular zinc and independent of intracellular calcium in lymphocytes. Exp Cell Res. 1994 Apr;211(2):339–343. doi: 10.1006/excr.1994.1096. [DOI] [PubMed] [Google Scholar]
- Vadas P., Stefanski E., Pruzanski W. Potential therapeutic efficacy of inhibitors of human phospholipase A2 in septic shock. Agents Actions. 1986 Nov;19(3-4):194–202. doi: 10.1007/BF01966206. [DOI] [PubMed] [Google Scholar]
- Vázquez A., Tudela J., Varón R., García-Cánovas F. A kinetic study of the generation and decomposition of some phenothiazine free radicals formed during enzymatic oxidation of phenothiazines by peroxidase-hydrogen peroxide. Biochem Pharmacol. 1992 Sep 1;44(5):889–894. doi: 10.1016/0006-2952(92)90120-8. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]