Abstract
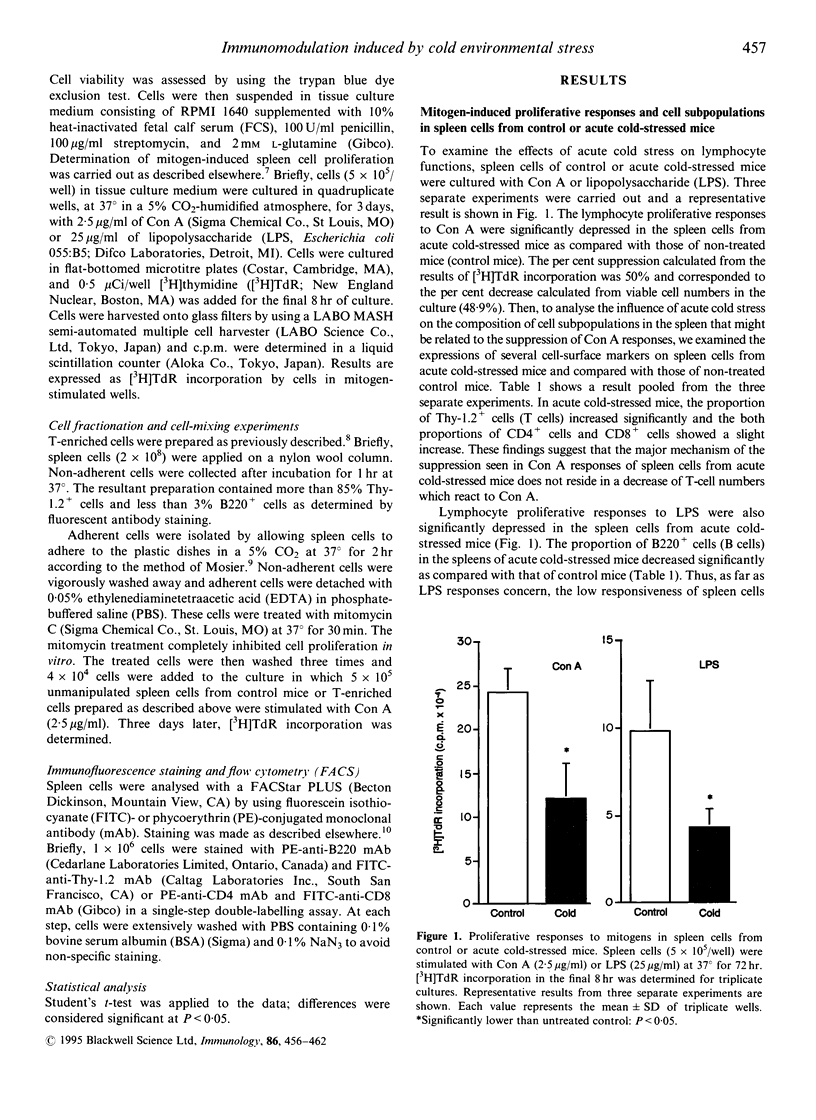
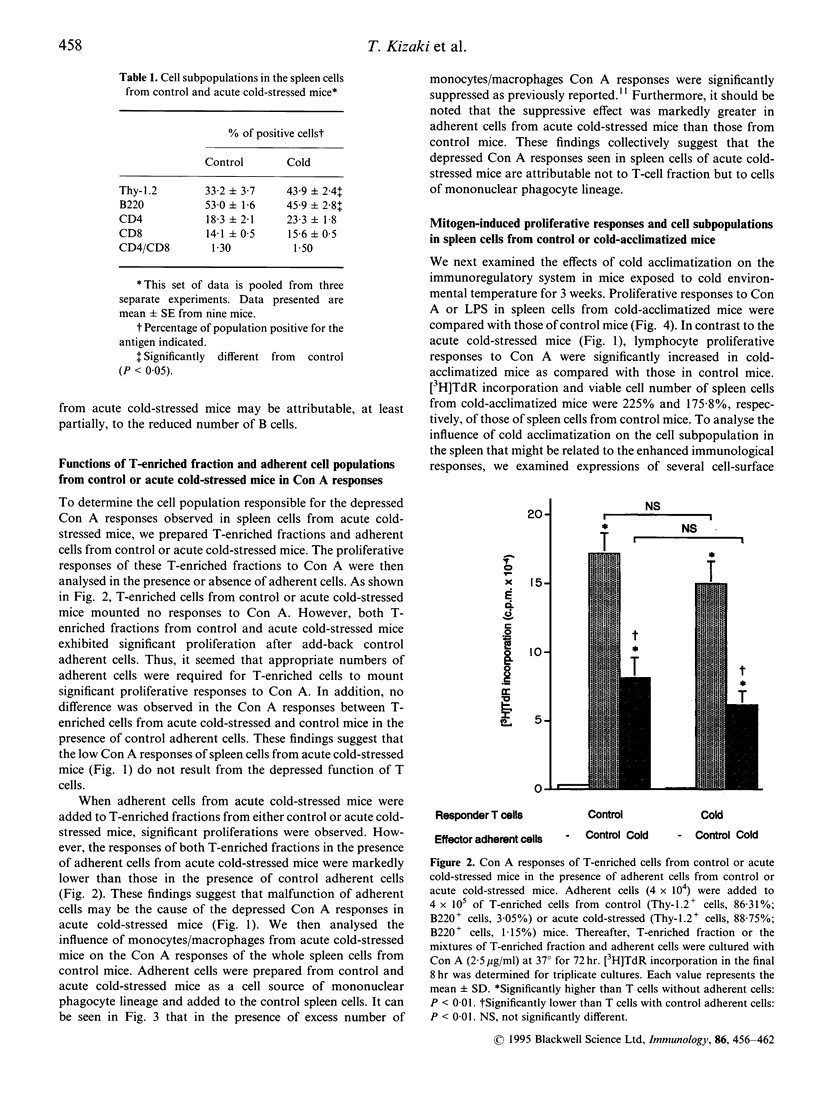
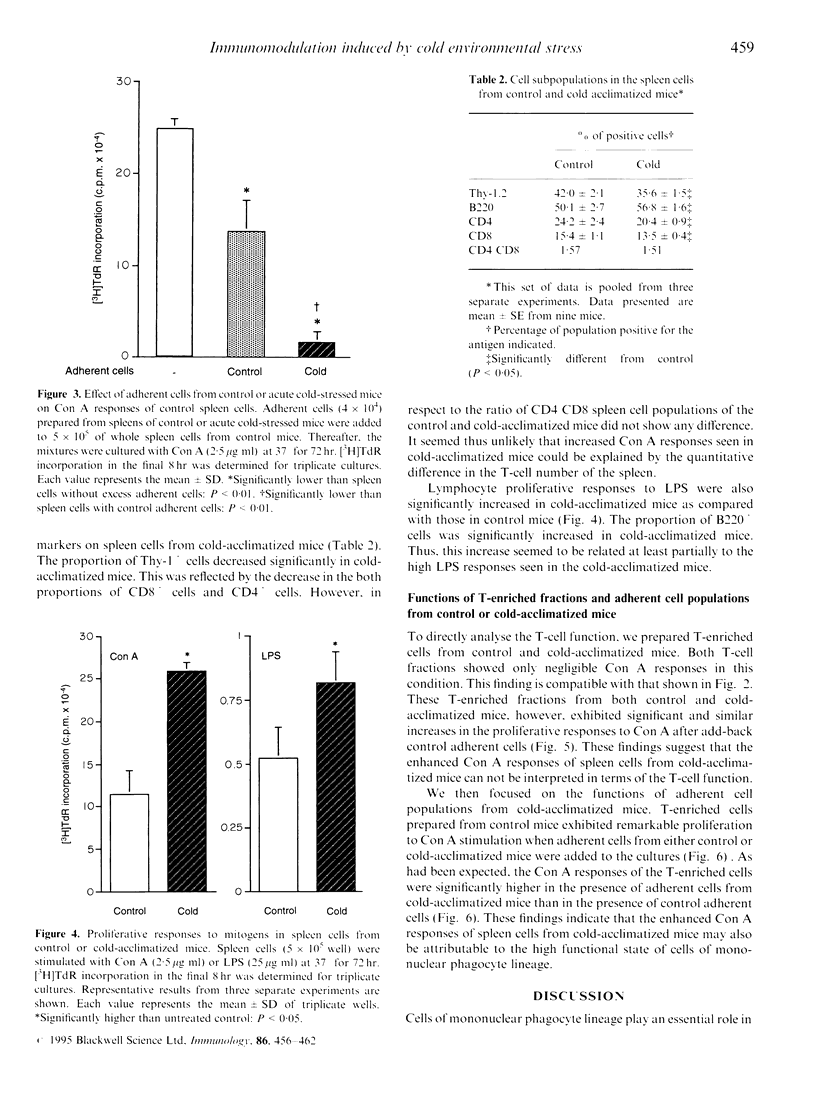
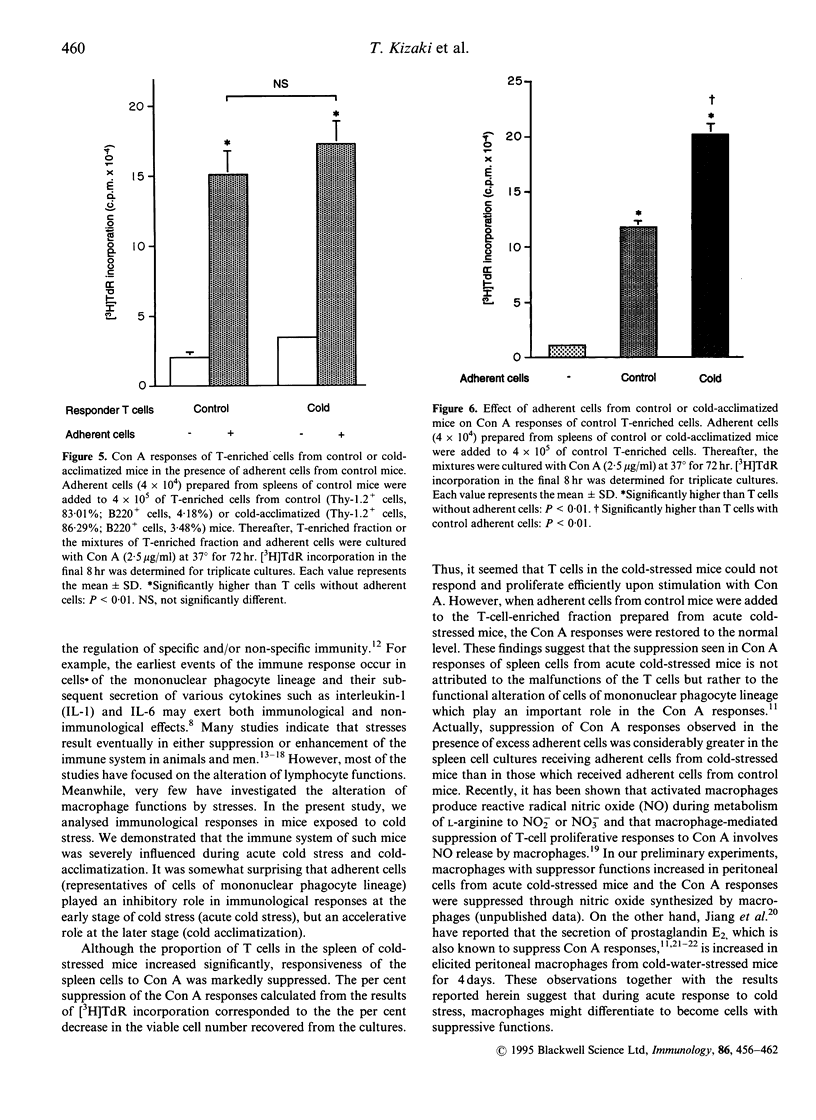
Immunoregulatory states in acute cold-stressed or cold-acclimatized mice were investigated. When male C57BL/6 mice were exposed to environmental temperature of 5 degrees for 24 hr (acute cold stress), the spleen cells showed depressed proliferative responses to stimulation with concanavalin A (Con A) or lipopolysaccharide (LPS) compared with control mice (reared at 25 degrees). The proportion of Thy-1.2+ cells increased significantly in spleens from these acute cold-stressed mice. The Con A responses of T-enriched cells from acute cold-stressed mice were restored to the normal level by adding plastic-adherent cells from control mice. Further, adherent cells from acute cold-stressed mice markedly suppressed the Con A responses of control spleen cells. Thus, the plastic-adherent cells appeared to be responsible for the suppressed Con A responses. On the other hand, proliferative responses to Con A or LPS were elevated in spleen cells from mice exposed to 5 degrees for 3 weeks (cold acclimatization). A significant decrease of Thy-1.2+ cells was detected in these spleens. It was shown that the enhanced proliferative responses were attributable to functional alterations of the plastic adherent cell population but not to those of lymphoid cell population. These findings indicate that the low or high responsiveness of spleen cells to Con A observed in cold-stressed or cold-acclimatized mice, respectively, may be due to a mechanism including the contrary modulations of functions of cells of mononuclear phagocyte lineage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Ben-Nathan D., Lustig S., Feuerstein G. The influence of cold or isolation stress on neuroinvasiveness and virulence of an attenuated variant of West Nile virus. Arch Virol. 1989;109(1-2):1–10. doi: 10.1007/BF01310513. [DOI] [PubMed] [Google Scholar]
- Blalock J. E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989 Jan;69(1):1–32. doi: 10.1152/physrev.1989.69.1.1. [DOI] [PubMed] [Google Scholar]
- Cheng G. J., Morrow-Tesch J. L., Beller D. I., Levy E. M., Black P. H. Immunosuppression in mice induced by cold water stress. Brain Behav Immun. 1990 Dec;4(4):278–291. doi: 10.1016/0889-1591(90)90032-l. [DOI] [PubMed] [Google Scholar]
- Chowers I., Conforti N., Feldman S. Effect of changing levels of glucocorticosteroids on body temperature on exposure to cold. Am J Physiol. 1970 Jun;218(6):1563–1567. doi: 10.1152/ajplegacy.1970.218.6.1563. [DOI] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Collins K. J., Weiner J. S. Endocrinological aspects of exposure to high environmental temperatures. Physiol Rev. 1968 Oct;48(4):785–839. doi: 10.1152/physrev.1968.48.4.785. [DOI] [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- De Souza E. B. Corticotropin-releasing factor and interleukin-1 receptors in the brain-endocrine-immune axis. Role in stress response and infection. Ann N Y Acad Sci. 1993 Oct 29;697:9–27. doi: 10.1111/j.1749-6632.1993.tb49919.x. [DOI] [PubMed] [Google Scholar]
- Fujiwara R., Orita K. The enhancement of the immune response by pain stimulation in mice. I. The enhancement effect on PFC production via sympathetic nervous system in vivo and in vitro. J Immunol. 1987 Jun 1;138(11):3699–3703. [PubMed] [Google Scholar]
- Girard M. T., Hjaltadottir S., Fejes-Toth A. N., Guyre P. M. Glucocorticoids enhance the gamma-interferon augmentation of human monocyte immunoglobulin G Fc receptor expression. J Immunol. 1987 May 15;138(10):3235–3241. [PubMed] [Google Scholar]
- Gisler R. H. Stress and the hormonal regulation of the immune response in mice. Psychother Psychosom. 1974;23(1-6):197–208. doi: 10.1159/000286643. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Sugano T., Shimada M., Tatsumi H. Changes in excretion of catecholamines and metabolites during acclimation to cold. Nihon Seirigaku Zasshi. 1978;40(2):39–41. [PubMed] [Google Scholar]
- Hayashi O., Kikuchi M. Time relationship between ambient temperature change and antigen stimulation on immune responses of mice. Int J Biometeorol. 1989 Jan;33(1):19–23. doi: 10.1007/BF01045892. [DOI] [PubMed] [Google Scholar]
- Helmberg A., Fässler R., Geley S., Jöhrer K., Kroemer G., Böck G., Kofler R. Glucocorticoid-regulated gene expression in the immune system. Analysis of glucocorticoid-regulated transcripts from the mouse macrophage-like cell line P388D1. J Immunol. 1990 Dec 15;145(12):4332–4337. [PubMed] [Google Scholar]
- Huebert T., Evans W. S., Hardy M. Hymenolepis diminuta: the effect of cold temperature exposure on infections in mice. Exp Parasitol. 1990 May;70(4):398–403. doi: 10.1016/0014-4894(90)90123-t. [DOI] [PubMed] [Google Scholar]
- Jefferies W. M. Cortisol and immunity. Med Hypotheses. 1991 Mar;34(3):198–208. doi: 10.1016/0306-9877(91)90212-h. [DOI] [PubMed] [Google Scholar]
- Kizaki T., Ishige M., Bingyan W., Day N. K., Good R. A., Onoé K. Generation of CD8+ suppressor T cells by protoscoleces of Echinococcus multilocularis in vitro. Immunology. 1993 Jul;79(3):412–417. [PMC free article] [PubMed] [Google Scholar]
- Kizaki T., Ishige M., Bingyan W., Kumagai M., Day N. K., Good R. A., Onoé K. Interleukin-1-dependent mitogenic responses induced by protoscoleces of Echinococcus multilocularis in murine lymphocytes. J Leukoc Biol. 1993 Mar;53(3):233–239. doi: 10.1002/jlb.53.3.233. [DOI] [PubMed] [Google Scholar]
- Kizaki T., Kobayashi S., Ogasawara K., Day N. K., Good R. A., Onoé K. Immune suppression induced by protoscoleces of Echinococcus multilocularis in mice. Evidence for the presence of CD8dull suppressor cells in spleens of mice intraperitoneally infected with E. multilocularis. J Immunol. 1991 Sep 1;147(5):1659–1666. [PubMed] [Google Scholar]
- Kusnecov A. V., Grota L. J., Schmidt S. G., Bonneau R. H., Sheridan J. F., Glaser R., Moynihan J. A. Decreased herpes simplex viral immunity and enhanced pathogenesis following stressor administration in mice. J Neuroimmunol. 1992 May;38(1-2):129–137. doi: 10.1016/0165-5728(92)90097-5. [DOI] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Ohno H., Yahata T., Yamashita K., Kuroshima A. Effect of acute cold exposure on ACTH and zinc concentrations in human plasma. Jpn J Physiol. 1987;37(4):749–755. doi: 10.2170/jjphysiol.37.749. [DOI] [PubMed] [Google Scholar]
- Okimura T., Ogawa M., Yamauchi T. Stress and immune responses. III. Effect of restraint stress on delayed type hypersensitivity (DTH) response, natural killer (NK) activity and phagocytosis in mice. Jpn J Pharmacol. 1986 Jun;41(2):229–235. doi: 10.1254/jjp.41.229. [DOI] [PubMed] [Google Scholar]
- Sabiston B. H., Rose J. E. Effect of cold exposure on the metabolism of immunoglobulins in rabbits. J Immunol. 1976 Jan;116(1):106–111. [PubMed] [Google Scholar]
- Schleifer K. W., Mansfield J. M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993 Nov 15;151(10):5492–5503. [PubMed] [Google Scholar]
- Sheridan J. F., Feng N. G., Bonneau R. H., Allen C. M., Huneycutt B. S., Glaser R. Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J Neuroimmunol. 1991 Mar;31(3):245–255. doi: 10.1016/0165-5728(91)90046-a. [DOI] [PubMed] [Google Scholar]
- St Rose J. E., Sabiston B. H. Effect of cold exposure on the immunologic response of rabbits to human serum albumin. J Immunol. 1971 Aug;107(2):339–343. [PubMed] [Google Scholar]
- Thompson J., van Furth R. The effect of glucocorticosteroids on the proliferation and kinetics of promonocytes and monocytes of the bone marrow. J Exp Med. 1973 Jan 1;137(1):10–21. doi: 10.1084/jem.137.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Warren M. K., Vogel S. N. Opposing effects of glucocorticoids on interferon-gamma-induced murine macrophage Fc receptor and Ia antigen expression. J Immunol. 1985 Apr;134(4):2462–2469. [PubMed] [Google Scholar]
- Xu Y., Yang Z., Su C. Enhancement of cellular immune function during cold adaptation of BALB/c inbred mice. Cryobiology. 1992 Jun;29(3):422–427. doi: 10.1016/0011-2240(92)90044-3. [DOI] [PubMed] [Google Scholar]
- Zwilling B. S., Brown D., Feng N., Sheridan J., Pearl D. The effect of adrenalectomy on the restraint stressed induced suppression of MHC class II expression by murine peritoneal macrophages. Brain Behav Immun. 1993 Mar;7(1):29–35. doi: 10.1006/brbi.1993.1003. [DOI] [PubMed] [Google Scholar]
- Zwilling B. S., Dinkins M., Christner R., Faris M., Griffin A., Hilburger M., McPeek M., Pearl D. Restraint stress-induced suppression of major histocompatibility complex class II expression by murine peritoneal macrophages. J Neuroimmunol. 1990 Sep-Oct;29(1-3):125–130. doi: 10.1016/0165-5728(90)90154-f. [DOI] [PubMed] [Google Scholar]


