Abstract
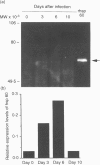
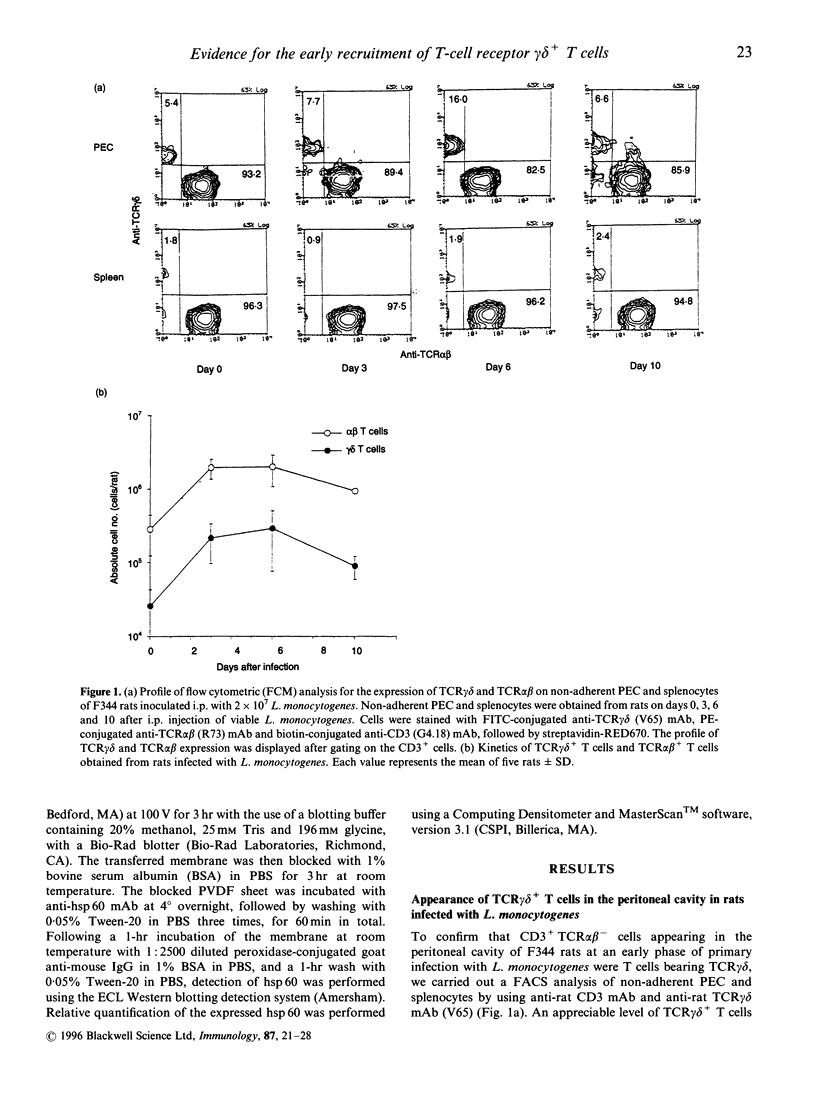
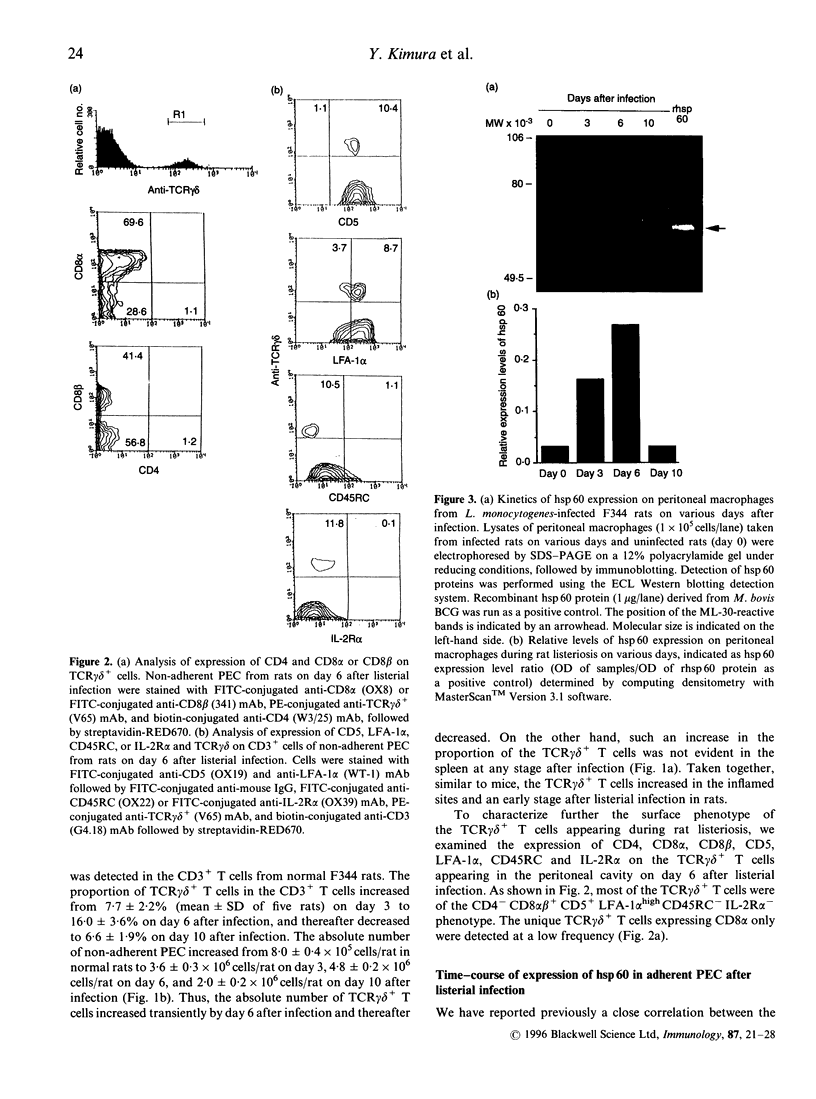
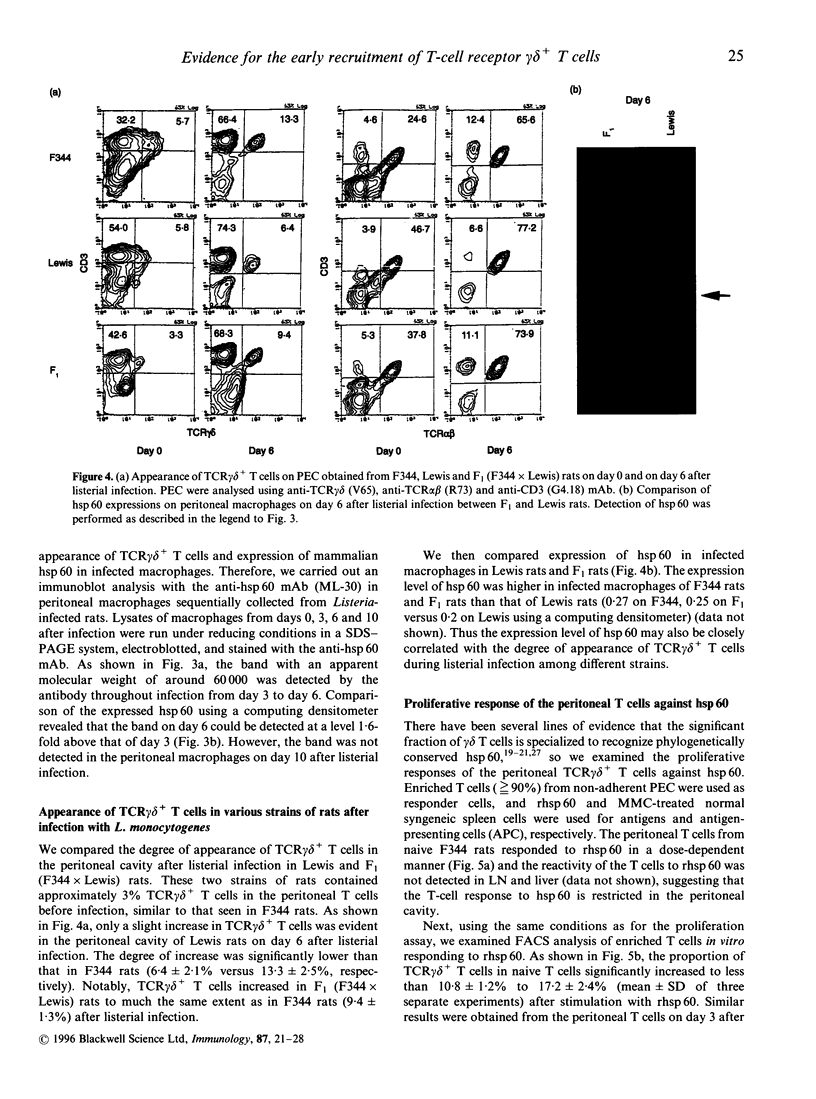
We have previously reported that heat-shock protein (hsp) 60-reactive T-cell receptor (TCR)gamma delta+ T cells appear in the peritoneal cavity during the early stage of infection with Listeria monocytogenes in mice. In this study, we examined the kinetics of TCR gamma delta+ T cells during listeriosis in F344 rats by flow cytometry using a V65 monoclonal antibody (mAb) directed to a constant determinant of rat TCR gamma delta chains. TCR gamma delta+ T cells significantly increased in the peritoneal cavity on day 6 and then decreased by day 10 after infection, in parallel with the kinetics of hsp60 expression in the peritoneal macrophages during listeriosis in F344 rats. Most of the early appearing TCR gamma delta+ T cells were of the CD4- CD8 alpha beta+ CD5+ lymphocyte function-associated antigen (LFA)-1 alpha high CD45RC- interleukin-2 receptor (IL-2R) alpha- phenotype, although a significant fraction of the TCR gamma delta+ T cells expressed CD8 alpha only. The increase in TCR gamma delta+ T cells during listeriosis was prominent in F1 (F344 x Lewis) rats but only marginal in Lewis rats, which was correlated with the expression level of hsp 60 in the peritoneal macrophages. The peritoneal TCR gamma delta+ T cells in naive F344 rats appeared to proliferate significantly in response to recombinant hsp 60 (rhsp 60) derived from Mycobacterium bovis bacillus Calmette-Guérin (BCG). These results imply that the early appearance of hsp 60-reactive TCR gamma delta+ T cells during listerial infection can be generalized across species.
Full text
PDF

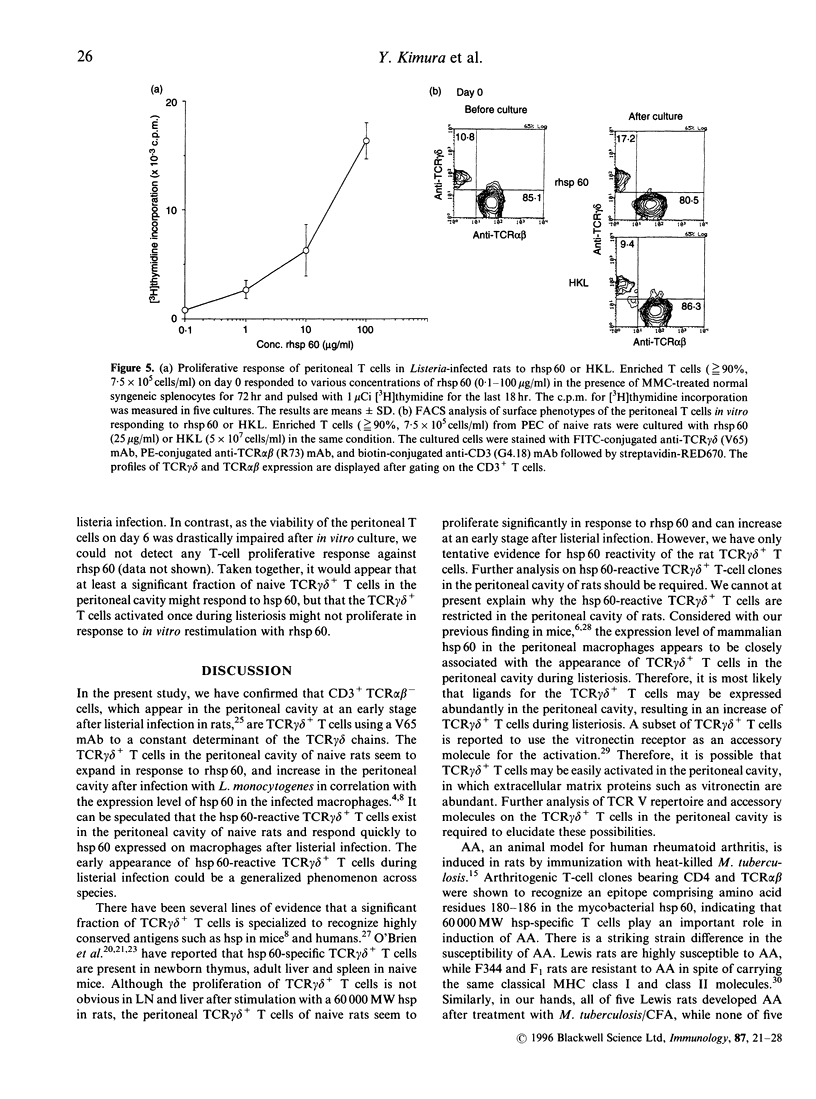


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton S. M., van der Zee R., Noordzij A., van Eden W. Differential mycobacterial 65-kDa heat shock protein T cell epitope recognition after adjuvant arthritis-inducing or protective immunization protocols. J Immunol. 1994 Apr 1;152(7):3656–3664. [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Chung I. Y., Norris J. G., Benveniste E. N. Differential tumor necrosis factor alpha expression by astrocytes from experimental allergic encephalomyelitis-susceptible and -resistant rat strains. J Exp Med. 1991 Apr 1;173(4):801–811. doi: 10.1084/jem.173.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Emoto M., Danbara H., Yoshikai Y. Induction of gamma/delta T cells in murine salmonellosis by an avirulent but not by a virulent strain of Salmonella choleraesuis. J Exp Med. 1992 Aug 1;176(2):363–372. doi: 10.1084/jem.176.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto M., Naito T., Nakamura R., Yoshikai Y. Different appearance of gamma delta T cells during salmonellosis between Ityr and Itys mice. J Immunol. 1993 Apr 15;150(8 Pt 1):3411–3420. [PubMed] [Google Scholar]
- Fisch P., Malkovsky M., Kovats S., Sturm E., Braakman E., Klein B. S., Voss S. D., Morrissey L. W., DeMars R., Welch W. J. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science. 1990 Nov 30;250(4985):1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- Fu Y. X., Roark C. E., Kelly K., Drevets D., Campbell P., O'Brien R., Born W. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol. 1994 Oct 1;153(7):3101–3115. [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Harada M., Matsuzaki G., Yoshikai Y., Kobayashi N., Kurosawa S., Takimoto H., Nomoto K. Autoreactive and heat shock protein 60-recognizing CD4+ T-cells show antitumor activity against syngeneic fibrosarcoma. Cancer Res. 1993 Jan 1;53(1):106–111. [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Tanaka T., Yoshikai Y. The appearance and role of gamma delta T cells in the peritoneal cavity and liver during primary infection with Listeria monocytogenes in rats. Int Immunol. 1992 Oct;4(10):1129–1136. doi: 10.1093/intimm/4.10.1129. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Yoshikai Y., Matsuzaki G., Nomoto K. Early appearing gamma/delta-bearing T cells during infection with Calmétte Guérin bacillus. J Immunol. 1991 Apr 15;146(8):2754–2762. [PubMed] [Google Scholar]
- Kaufmann S. H., Schoel B., Wand-Württenberger A., Steinhoff U., Munk M. E., Koga T. T-cells, stress proteins, and pathogenesis of mycobacterial infections. Curr Top Microbiol Immunol. 1990;155:125–141. doi: 10.1007/978-3-642-74983-4_9. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Schoel B., van Embden J. D., Koga T., Wand-Württenberger A., Munk M. E., Steinhoff U. Heat-shock protein 60: implications for pathogenesis of and protection against bacterial infections. Immunol Rev. 1991 Jun;121:67–90. doi: 10.1111/j.1600-065x.1991.tb00823.x. [DOI] [PubMed] [Google Scholar]
- Kühnlein P., Park J. H., Herrmann T., Elbe A., Hünig T. Identification and characterization of rat gamma/delta T lymphocytes in peripheral lymphoid organs, small intestine, and skin with a monoclonal antibody to a constant determinant of the gamma/delta T cell receptor. J Immunol. 1994 Aug 1;153(3):979–986. [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Ogasawara T., Emoto M., Kiyotani K., Shimokata K., Yoshida T., Nagai Y., Yoshikai Y. Sendai virus pneumonia: evidence for the early recruitment of gamma delta T cells during the disease course. J Virol. 1994 Jun;68(6):4022–4027. doi: 10.1128/jvi.68.6.4022-4027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga S., Yoshikai Y., Takeda Y., Hiromatsu K., Nomoto K. Sequential appearance of gamma/delta- and alpha/beta-bearing T cells in the peritoneal cavity during an i.p. infection with Listeria monocytogenes. Eur J Immunol. 1990 Mar;20(3):533–538. doi: 10.1002/eji.1830200311. [DOI] [PubMed] [Google Scholar]
- Raine C. S. The Dale E. McFarlin Memorial Lecture: the immunology of the multiple sclerosis lesion. Ann Neurol. 1994;36 (Suppl):S61–S72. doi: 10.1002/ana.410360716. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. The structure, function, and molecular genetics of the gamma/delta T cell receptor. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- Roberts K., Yokoyama W. M., Kehn P. J., Shevach E. M. The vitronectin receptor serves as an accessory molecule for the activation of a subset of gamma/delta T cells. J Exp Med. 1991 Jan 1;173(1):231–240. doi: 10.1084/jem.173.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K., DeLeo A. B., Old L. J. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci U S A. 1986 May;83(10):3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida S., Hasegawa T., Takeuchi M., Niimi N., Ueda M., Kaneda T., Tanaka T., Tamatani T., Miyasaka M., Yoshikai Y. Intercellular adhesion molecule-1 and leukocyte function-associated antigen-1 are involved in protection mediated by CD3+TCR alpha beta- T cells at the early stage after infection with Listeria monocytogenes in rats. Int Immunol. 1994 Jul;6(7):955–961. doi: 10.1093/intimm/6.7.955. [DOI] [PubMed] [Google Scholar]
- Ullrich S. J., Robinson E. A., Law L. W., Willingham M., Appella E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc Natl Acad Sci U S A. 1986 May;83(10):3121–3125. doi: 10.1073/pnas.83.10.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Hogervorst E. J., Hensen E. J., van der Zee R., van Embden J. D., Cohen I. R. A cartilage-mimicking T-cell epitope on a 65K mycobacterial heat-shock protein: adjuvant arthritis as a model for human rheumatoid arthritis. Curr Top Microbiol Immunol. 1989;145:27–43. doi: 10.1007/978-3-642-74594-2_3. [DOI] [PubMed] [Google Scholar]