Abstract
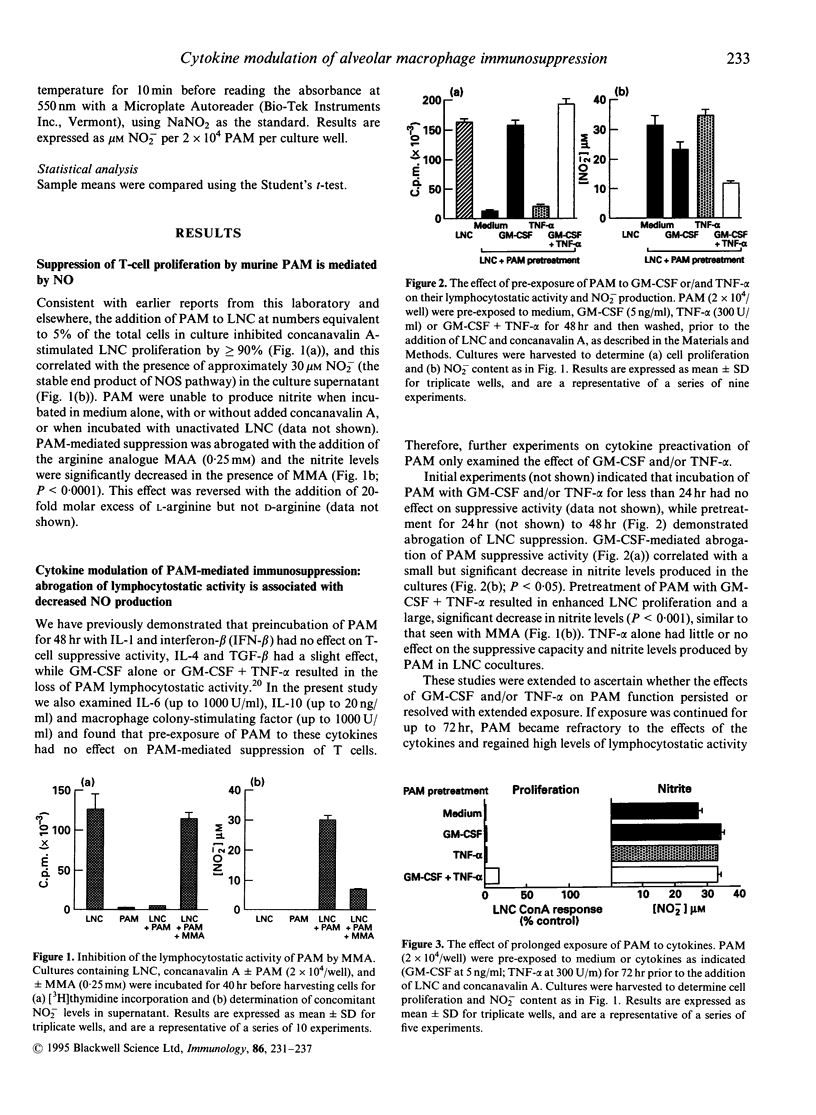
Under steady-state conditions, T-cell activation in the lung is tightly controlled by lymphocytostatic signals from resident pulmonary alveolar macrophages (PAM). The present study focuses upon the mechanism of suppression in the mouse, and how it is bypassed during local inflammatory challenge. Reactive nitrogen intermediates such as nitric oxide (NO) are shown to play a central role in the process as the expression of lymphocytostatic activity by resident murine PAM was abrogated by the NO synthetase inhibitor N-monomethyl-arginine. Overnight pretreatment of resident PAM with granulocyte-macrophage colony-stimulating factor (GM-CSF) abrogated lymphocytostatic activity, with a concomitant small decrease in NO production; this effect was markedly amplified by tumour necrosis factor-alpha (TNF-alpha), but the latter was ineffective alone. The cytokines were inactive if added singly or in combination to fresh PAM:T-cell co-cultures. If GM-CSF plus TNF-alpha exposure of PAM was prolonged beyond 48 hr, both lymphocytostatic and NO-producing capacity were spontaneously re-established. Transforming growth factor-beta (TGF-beta) also inhibited both NO production and lymphocytostatic activity of PAM, but in contrast to GM-CSF and TNF-alpha, TGF-beta was only active if present throughout the PAM:T-cell coculture period. Additionally, monocytes recruited into the lung by a sterile inflammatory stimulus are shown to be initially stimulatory towards T-cell activation, and to progressively develop both T-cell suppressive- and NO synthetic-capacity as they mature into mature PAM in vivo. Thus, during acute lung inflammation, a series of overlapping mechanisms are potentially available to bypass local immunosuppression: secretion of cytokines which are capable of temporarily abrogating the immunosuppressive activity of resident PAM, and the recruitment of permissive monocytes which exhibit potent accessory cell activity, the net result being the creation of a transient 'window' for induction of local T cell-mediated immunity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Becker S., Devlin R. B., Haskill J. S. Differential production of tumor necrosis factor, macrophage colony stimulating factor, and interleukin 1 by human alveolar macrophages. J Leukoc Biol. 1989 Apr;45(4):353–361. [PubMed] [Google Scholar]
- Bilyk N., Holt P. G. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med. 1993 Jun 1;177(6):1773–1777. doi: 10.1084/jem.177.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyk N., Holt P. G. The surface phenotypic characterization of lung macrophages in C3H/HeJ mice. Immunology. 1991 Dec;74(4):645–651. [PMC free article] [PubMed] [Google Scholar]
- Bilyk N., Mackenzie J. S., Papadimitriou J. M., Holt P. G. Functional studies on macrophage populations in the airways and the lung wall of SPF mice in the steady-state and during respiratory virus infection. Immunology. 1988 Nov;65(3):417–425. [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Alveolar macrophage response to carbon in monocyte-depleted mice. Am Rev Respir Dis. 1982 Oct;126(4):708–711. doi: 10.1164/arrd.1982.126.4.708. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Cox G. W., Melillo G., Chattopadhyay U., Mullet D., Fertel R. H., Varesio L. Tumor necrosis factor-alpha-dependent production of reactive nitrogen intermediates mediates IFN-gamma plus IL-2-induced murine macrophage tumoricidal activity. J Immunol. 1992 Nov 15;149(10):3290–3296. [PubMed] [Google Scholar]
- Cumberbatch M., Kimber I. Dermal tumour necrosis factor-alpha induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans' cell migration. Immunology. 1992 Feb;75(2):257–263. [PMC free article] [PubMed] [Google Scholar]
- Deng W., Thiel B., Tannenbaum C. S., Hamilton T. A., Stuehr D. J. Synergistic cooperation between T cell lymphokines for induction of the nitric oxide synthase gene in murine peritoneal macrophages. J Immunol. 1993 Jul 1;151(1):322–329. [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Fan K., Ruan Q., Sensenbrenner L., Chen B. D. Up-regulation of granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors in murine peritoneal exudate macrophages by both GM-CSF and IL-3. J Immunol. 1992 Jul 1;149(1):96–102. [PubMed] [Google Scholar]
- Fireman E., Ben Efraim S., Greif J., Alguetti A., Ayalon D., Topilsky M. Correlation between PGE2 production and suppressor activity of alveolar macrophages from patients with interstitial lung diseases. Immunol Lett. 1988 Jun;18(2):159–165. doi: 10.1016/0165-2478(88)90058-2. [DOI] [PubMed] [Google Scholar]
- Fu Y., Blankenhorn E. P. Nitric oxide-induced anti-mitogenic effects in high and low responder rat strains. J Immunol. 1992 Apr 1;148(7):2217–2222. [PubMed] [Google Scholar]
- Herscowitz H. B. In defense of the lung: paradoxical role of the pulmonary alveolar macrophage. Ann Allergy. 1985 Nov;55(5):634–650. [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. II. Inhibition of lymphocyte proliferation by purified macrophages from rat lung. Immunology. 1979 Jun;37(2):429–436. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. III. Studies on the mechanisms of inhibition of T-cell proliferation. Immunology. 1979 Jun;37(2):437–445. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986 Feb;63(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Inhibitory activity of unstimulated alveolar macrophages on T-lymphocyte blastogenic response. Am Rev Respir Dis. 1978 Oct;118(4):791–793. doi: 10.1164/arrd.1978.118.4.791. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Oliver J., Bilyk N., McMenamin C., McMenamin P. G., Kraal G., Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993 Feb 1;177(2):397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Fukuto J. M., Griscavage J. M., Rogers N. E., Byrns R. E. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T., Isobe K. I., Hasegawa Y., Nakashima I., Shimokata K. Immunosuppressive activity induced by nitric oxide in culture supernatant of activated rat alveolar macrophages. Immunology. 1992 May;76(1):72–78. [PMC free article] [PubMed] [Google Scholar]
- Khalil N., Bereznay O., Sporn M., Greenberg A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989 Sep 1;170(3):727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Heufler C., Kämpgen E., Schneeweiss D., Böck G., Schuler G. Tumor necrosis factor alpha maintains the viability of murine epidermal Langerhans cells in culture, but in contrast to granulocyte/macrophage colony-stimulating factor, without inducing their functional maturation. J Exp Med. 1990 Jan 1;171(1):159–171. doi: 10.1084/jem.171.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The J. Burns Amberson LECTURE The induction and expression of cell-mediated hypersensitivity in the lung. Am Rev Respir Dis. 1971 Dec;104(6):813–828. doi: 10.1164/arrd.1971.104.6.813. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Martin J. H., Edwards S. W. Changes in mechanisms of monocyte/macrophage-mediated cytotoxicity during culture. Reactive oxygen intermediates are involved in monocyte-mediated cytotoxicity, whereas reactive nitrogen intermediates are employed by macrophages in tumor cell killing. J Immunol. 1993 Apr 15;150(8 Pt 1):3478–3486. [PubMed] [Google Scholar]
- McCombs C. C., Michalski J. P., Westerfield B. T., Light R. W. Human alveolar macrophages suppress the proliferative response of peripheral blood lymphocytes. Chest. 1982 Sep;82(3):266–271. doi: 10.1378/chest.82.3.266. [DOI] [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Monick M., Glazier J., Hunninghake G. W. Human alveolar macrophages suppress interleukin-1 (IL-1) activity via the secretion of prostaglandin E2. Am Rev Respir Dis. 1987 Jan;135(1):72–77. doi: 10.1164/arrd.1987.135.1.72. [DOI] [PubMed] [Google Scholar]
- Moore S. A., Strieter R. M., Rolfe M. W., Standiford T. J., Burdick M. D., Kunkel S. L. Expression and regulation of human alveolar macrophage-derived interleukin-1 receptor antagonist. Am J Respir Cell Mol Biol. 1992 Jun;6(6):569–575. doi: 10.1165/ajrcmb/6.6.569. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Billiar T. R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993 Aug;54(2):171–178. [PubMed] [Google Scholar]
- Rich E. A., Cooper C., Toossi Z., Leonard M. L., Stucky R. M., Wiblin R. T., Ellner J. J. Requirement for cell-to-cell contact for the immunosuppressive activity of human alveolar macrophages. Am J Respir Cell Mol Biol. 1991 Mar;4(3):287–294. doi: 10.1165/ajrcmb/4.3.287. [DOI] [PubMed] [Google Scholar]
- Roth M. D., Golub S. H. Human pulmonary macrophages utilize prostaglandins and transforming growth factor beta 1 to suppress lymphocyte activation. J Leukoc Biol. 1993 Apr;53(4):366–371. doi: 10.1002/jlb.53.4.366. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Lee D. K., Lacy J., Coleman D. L. Rat tracheal epithelial cells produce granulocyte/macrophage colony-stimulating factor. Am J Respir Cell Mol Biol. 1990 Jan;2(1):59–68. doi: 10.1165/ajrcmb/2.1.59. [DOI] [PubMed] [Google Scholar]
- Spiteri M. A., Poulter L. W. Characterization of immune inducer and suppressor macrophages from the normal human lung. Clin Exp Immunol. 1991 Jan;83(1):157–162. doi: 10.1111/j.1365-2249.1991.tb05607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepen T., McMenamin C., Girn B., Kraal G., Holt P. G. Regulation of IgE production in pre-sensitized animals: in vivo elimination of alveolar macrophages preferentially increases IgE responses to inhaled allergen. Clin Exp Allergy. 1992 Dec;22(12):1107–1114. doi: 10.1111/j.1365-2222.1992.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Thepen T., Van Rooijen N., Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989 Aug 1;170(2):499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodovotz Y., Bogdan C., Paik J., Xie Q. W., Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993 Aug 1;178(2):605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner L. A., Holt P. G., Mayrhofer G. Alveolar macrophages. VI. Regulation of alveolar macrophage-mediated suppression of lymphocyte proliferation by a putative T cell. Immunology. 1981 Jan;42(1):137–147. [PMC free article] [PubMed] [Google Scholar]


