Abstract
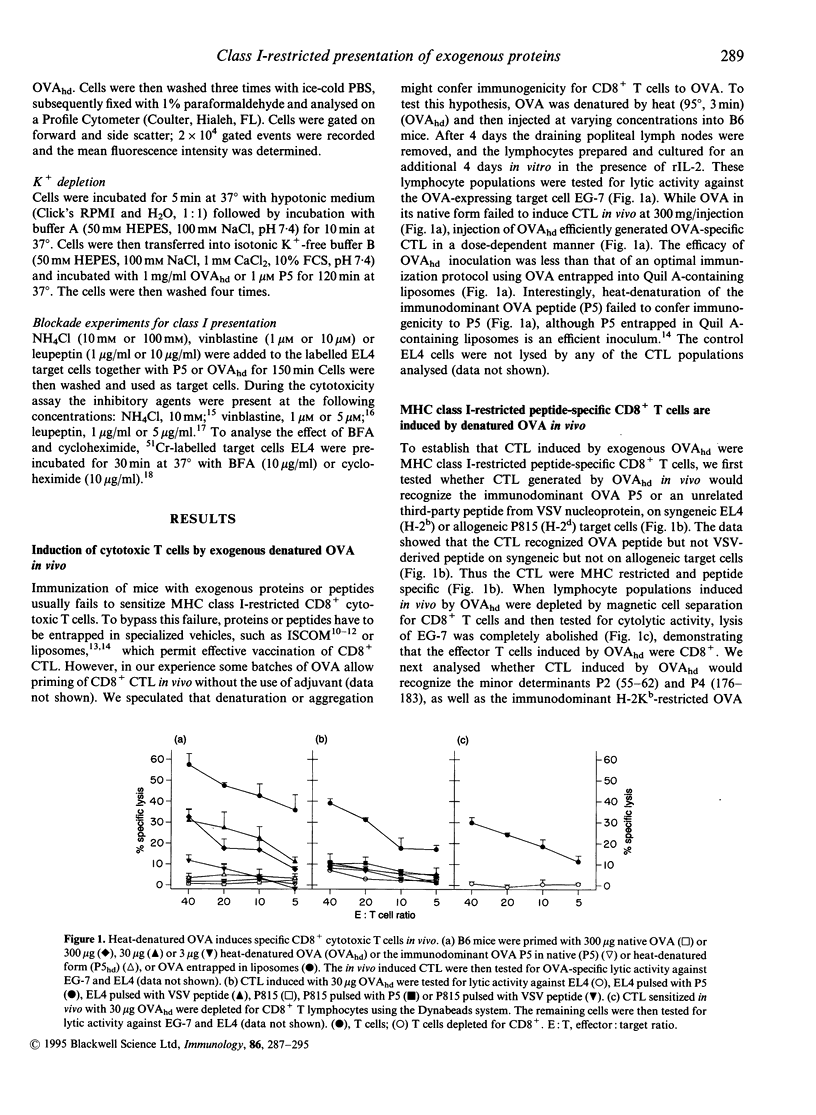
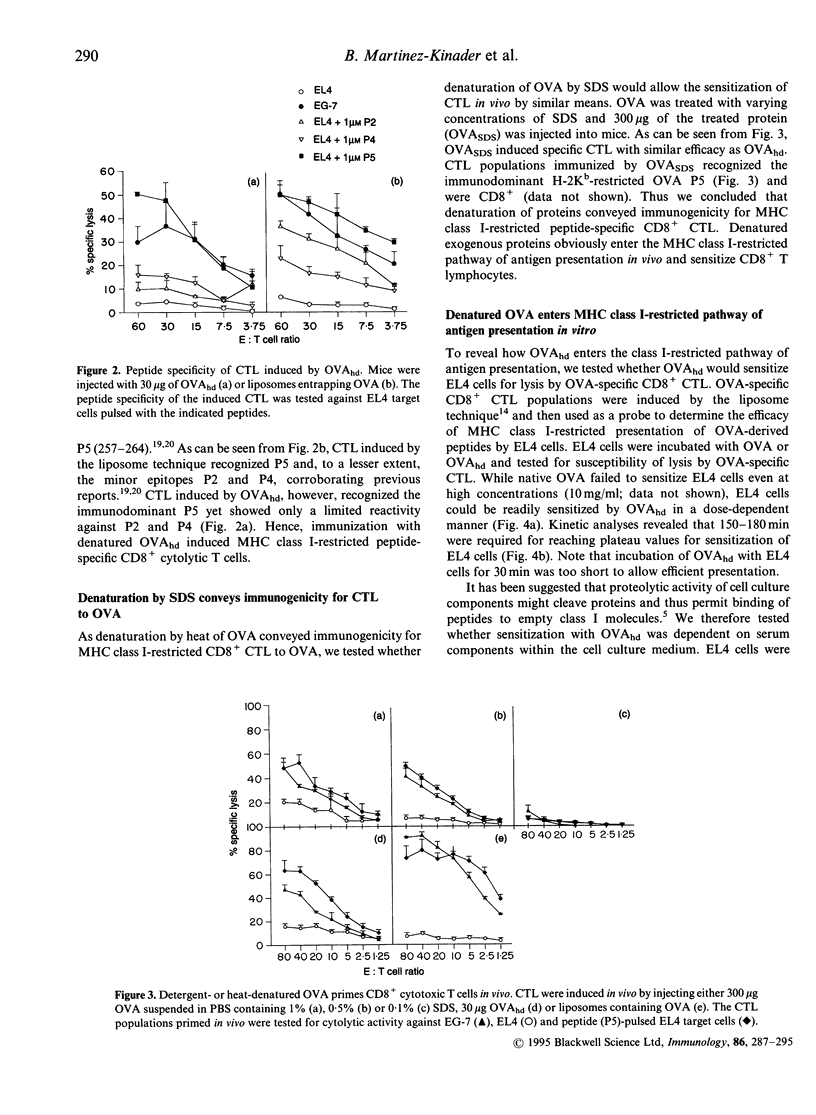
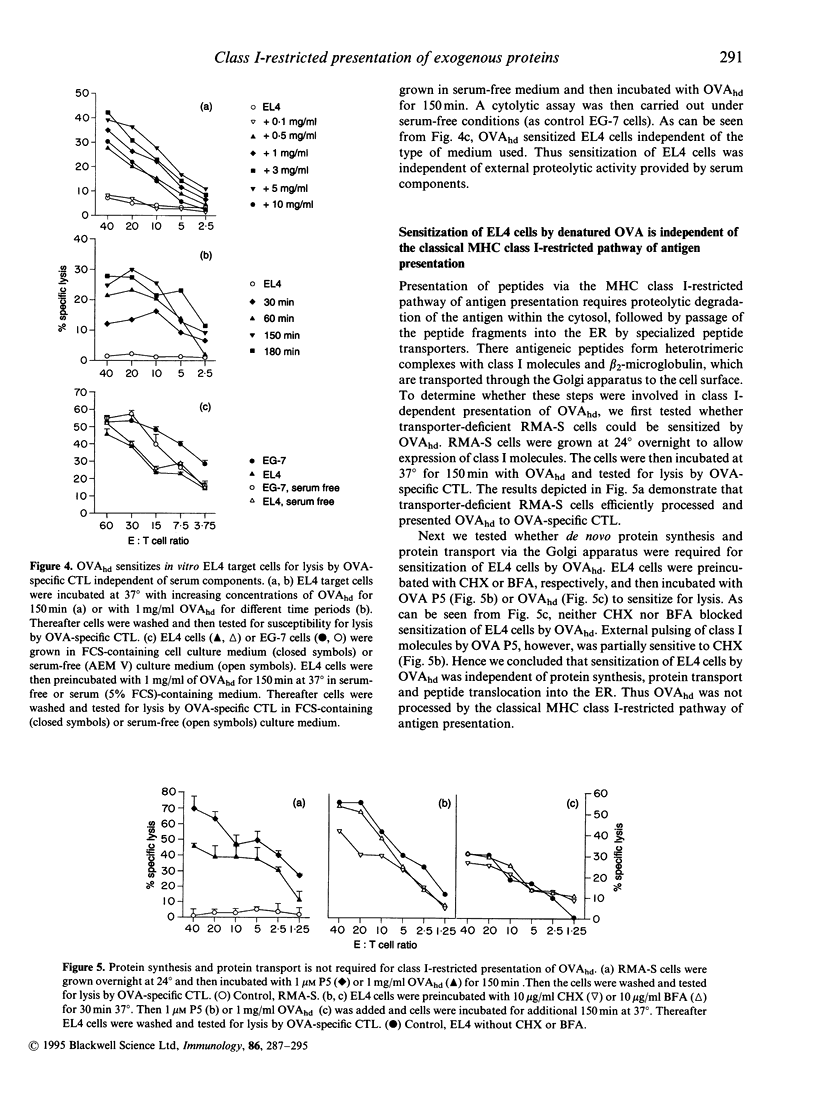
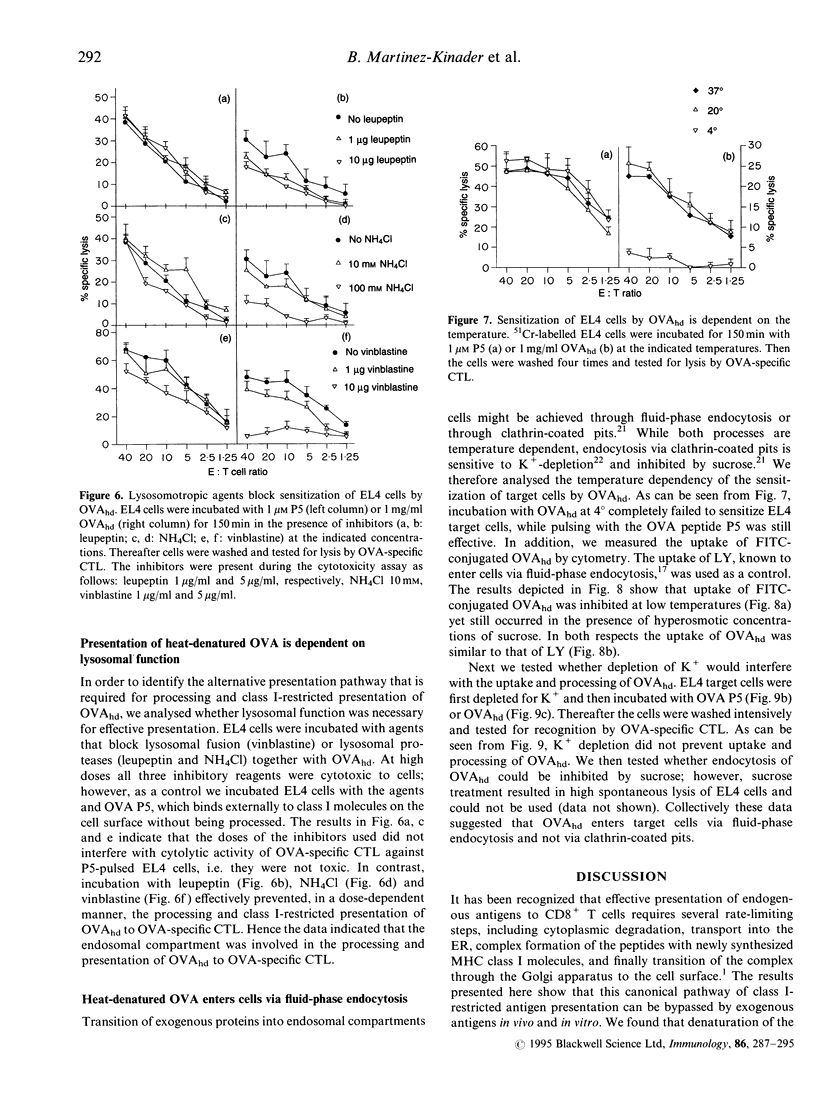
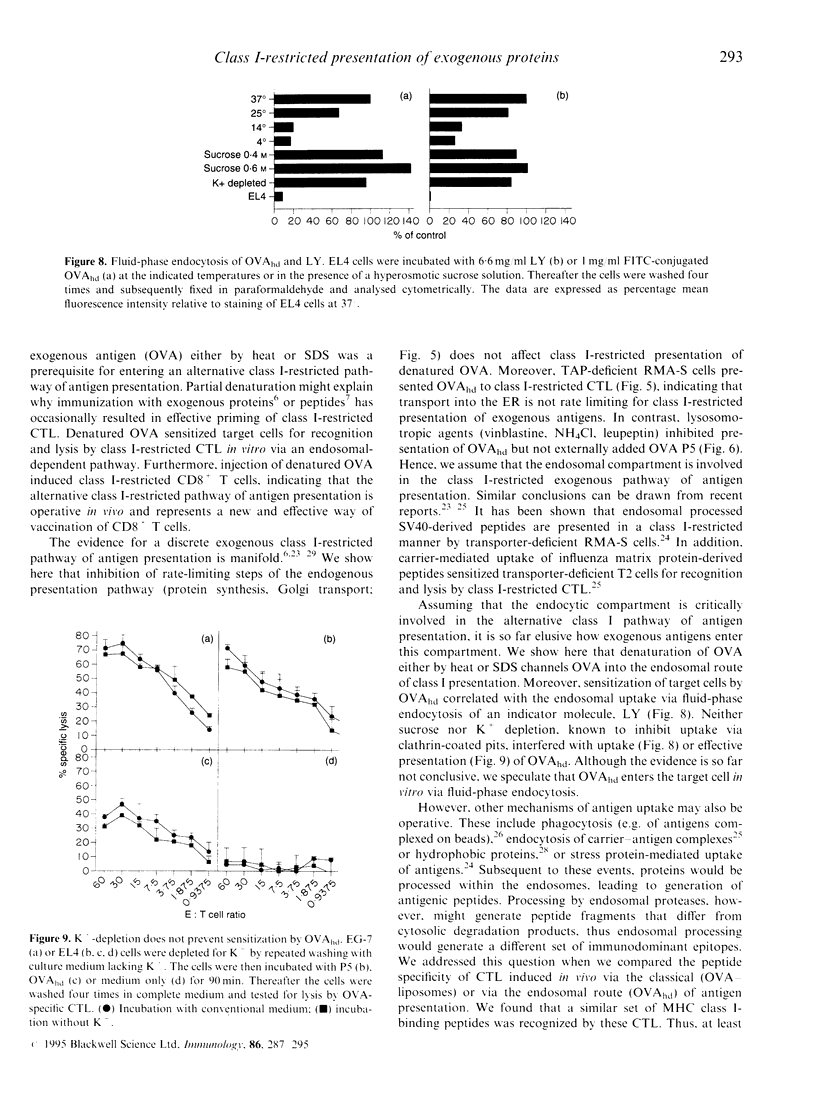
Immunization with exogenous proteins usually fails to immunize CD8+ T cells in vivo. Here we report that chicken ovalbumin (OVA) denatured by heat or sodium dodecyl sulphate (SDS) effectively induced CD8+ cytolytic T cells in vivo. The cytolytic T-lymphocyte (CTL) population generated recognized syngeneic target cells pulsed with the immunodominant OVA peptide (257-264) or transfected with the OVA protein-encoding gene. To analyse the mechanisms of how denatured OVA enters the class I-restricted pathway of antigen presentation, we took advantage of the fact that denatured OVA sensitizes target cells in vitro for lysis by OVA-specific CTL. We found that neither inhibition of protein synthesis (by cycloheximide) nor blocking of transport via the Golgi apparatus (by brefeldin A) interfered with the class I-restricted presentation of denatured OVA in vitro. In addition, transporter associated with antigen presentation (TAP)-dependent transport into the endoplasmic reticulum (ER) was not required for effective presentation, as TAP-deficient cells (RMA-S) could be sensitized effectively by denatured OVA for recognition by class I-restricted CTL. In contrast, class I-restricted presentation of denatured OVA was sensitive to lysosomotropic agents (NH4Cl, vinblastine and leupeptin), indicating that endosomal-like compartments are involved in the presentation of denatured OVA. Sensitization was inhibited at low temperature, yet took place in the presence of sucrose and in the absence of K+, indicating that denatured OVA enters the cell via fluid-phase endocytosis. Hence the results provide further evidence for an alternative class I-restricted pathway of antigen presentation for exogenous proteins. As that pathway seems to be effective in vivo, it offers a new and effective way of vaccination of CD8+ CTL.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel Motal U. M., Zhou X., Joki A., Siddiqi A. R., Srinivasa B. R., Stenvall K., Dahmén J., Jondal M. Major histocompatibility complex class I-binding peptides are recycled to the cell surface after internalization. Eur J Immunol. 1993 Dec;23(12):3224–3229. doi: 10.1002/eji.1830231227. [DOI] [PubMed] [Google Scholar]
- Abdel Motal U. M., Zhou X., Joki A., Siddiqi A. R., Srinivasa B. R., Stenvall K., Dahmén J., Jondal M. Major histocompatibility complex class I-binding peptides are recycled to the cell surface after internalization. Eur J Immunol. 1993 Dec;23(12):3224–3229. doi: 10.1002/eji.1830231227. [DOI] [PubMed] [Google Scholar]
- Brander C., Wyss-Coray T., Mauri D., Bettens F., Pichler W. J. Carrier-mediated uptake and presentation of a major histocompatibility complex class I-restricted peptide. Eur J Immunol. 1993 Dec;23(12):3217–3223. doi: 10.1002/eji.1830231226. [DOI] [PubMed] [Google Scholar]
- Capps G. G., Van Kampen M., Ward C. L., Zúiga M. C. Endocytosis of the class I major histocompatibility antigen via a phorbol myristate acetate-inducible pathway is a cell-specific phenomenon and requires the cytoplasmic domain. J Cell Biol. 1989 Apr;108(4):1317–1329. doi: 10.1083/jcb.108.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F. R., Bevan M. J. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989 Mar 1;169(3):603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Khilko S., Fecondo J., Margulies D. H., McCluskey J. Determinant selection of major histocompatibility complex class I-restricted antigenic peptides is explained by class I-peptide affinity and is strongly influenced by nondominant anchor residues. J Exp Med. 1994 Oct 1;180(4):1471–1483. doi: 10.1084/jem.180.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daukas G., Zigmond S. H. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol. 1985 Nov;101(5 Pt 1):1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Dick L. R., Aldrich C., Jameson S. C., Moomaw C. R., Pramanik B. C., Doyle C. K., DeMartino G. N., Bevan M. J., Forman J. M., Slaughter C. A. Proteolytic processing of ovalbumin and beta-galactosidase by the proteasome to a yield antigenic peptides. J Immunol. 1994 Apr 15;152(8):3884–3894. [PMC free article] [PubMed] [Google Scholar]
- Germain R. N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994 Jan 28;76(2):287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Gordon P. B., Høyvik H., Seglen P. O. Prelysosomal and lysosomal connections between autophagy and endocytosis. Biochem J. 1992 Apr 15;283(Pt 2):361–369. doi: 10.1042/bj2830361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg K., Kuon W., Wagner H. Vaccination of class I major histocompatibility complex (MHC)-restricted murine CD8+ cytotoxic T lymphocytes towards soluble antigens: immunostimulating-ovalbumin complexes enter the class I MHC-restricted antigen pathway and allow sensitization against the immunodominant peptide. Eur J Immunol. 1991 Jun;21(6):1523–1527. doi: 10.1002/eji.1830210628. [DOI] [PubMed] [Google Scholar]
- Hochman J. H., Jiang H., Matyus L., Edidin M., Pernis B. Endocytosis and dissociation of class I MHC molecules labeled with fluorescent beta-2 microglobulin. J Immunol. 1991 Mar 15;146(6):1862–1867. [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Clark K., Benacerraf B., Rock K. L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Rock K. L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995 Jan 13;267(5195):243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Larkin J. M., Brown M. S., Goldstein J. L., Anderson R. G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983 May;33(1):273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Lipford G. B., Hoffman M., Wagner H., Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J Immunol. 1993 Feb 15;150(4):1212–1222. [PubMed] [Google Scholar]
- Lipford G. B., Wagner H., Heeg K. Vaccination with immunodominant peptides encapsulated in Quil A-containing liposomes induces peptide-specific primary CD8+ cytotoxic T cells. Vaccine. 1994 Jan;12(1):73–80. doi: 10.1016/0264-410x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., Cho S., Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990 Dec 21;250(4988):1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Donachie A. M., Reid G., Jarrett O. Immune-stimulating complexes containing Quil A and protein antigen prime class I MHC-restricted T lymphocytes in vivo and are immunogenic by the oral route. Immunology. 1991 Mar;72(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- Nair S., Zhou X., Huang L., Rouse B. T. Class I restricted CTL recognition of a soluble protein delivered by liposomes containing lipophilic polylysines. J Immunol Methods. 1992 Aug 10;152(2):237–243. doi: 10.1016/0022-1759(92)90145-j. [DOI] [PubMed] [Google Scholar]
- Nuchtern J. G., Bonifacino J. S., Biddison W. E., Klausner R. D. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature. 1989 May 18;339(6221):223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- Otten G. R., Bikoff E., Ribaudo R. K., Kozlowski S., Margulies D. H., Germain R. N. Peptide and beta 2-microglobulin regulation of cell surface MHC class I conformation and expression. J Immunol. 1992 Jun 15;148(12):3723–3732. [PubMed] [Google Scholar]
- Reid P. A., Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990 Aug 16;346(6285):655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Gapud C. P., Keil W. Newly synthesized class II MHC chains are required for VSV G presentation to CTL clones. Cell Immunol. 1992 Jan;139(1):229–238. doi: 10.1016/0008-8749(92)90115-6. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L., Gramm C., Benacerraf B. Dissociation of beta 2-microglobulin leads to the accumulation of a substantial pool of inactive class I MHC heavy chains on the cell surface. Cell. 1991 May 17;65(4):611–620. doi: 10.1016/0092-8674(91)90093-e. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Melber K., Kuhröber A., Janowicz Z. A., Reimann J. Immunization with soluble hepatitis B virus surface protein elicits murine H-2 class I-restricted CD8+ cytotoxic T lymphocyte responses in vivo. J Immunol. 1994 Feb 1;152(3):1110–1119. [PubMed] [Google Scholar]
- Schirmbeck R., Reimann J. Peptide transporter-independent, stress protein-mediated endosomal processing of endogenous protein antigens for major histocompatibility complex class I presentation. Eur J Immunol. 1994 Jul;24(7):1478–1486. doi: 10.1002/eji.1830240704. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., Zerrahn J., Kuhröber A., Kury E., Deppert W., Reimann J. Immunization with soluble simian virus 40 large T antigen induces a specific response of CD3+ CD4- CD8+ cytotoxic T lymphocytes in mice. Eur J Immunol. 1992 Mar;22(3):759–766. doi: 10.1002/eji.1830220320. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Karasuyama H., Garner A. M. Cytotoxic T lymphocytes against a soluble protein. Nature. 1987 Oct 1;329(6138):449–451. doi: 10.1038/329449a0. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Takeshita T., Morein B., Putney S., Germain R. N., Berzofsky J. A. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990 Apr 26;344(6269):873–875. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- Weidt G., Utermöhlen O., Zerrahn J., Reimann J., Deppert W., Lehmann-Grube F. CD8+ T lymphocyte-mediated antiviral immunity in mice as a result of injection of recombinant viral proteins. J Immunol. 1994 Sep 15;153(6):2554–2561. [PubMed] [Google Scholar]
- Zhou F., Rouse B. T., Huang L. Prolonged survival of thymoma-bearing mice after vaccination with a soluble protein antigen entrapped in liposomes: a model study. Cancer Res. 1992 Nov 15;52(22):6287–6291. [PubMed] [Google Scholar]


