Abstract
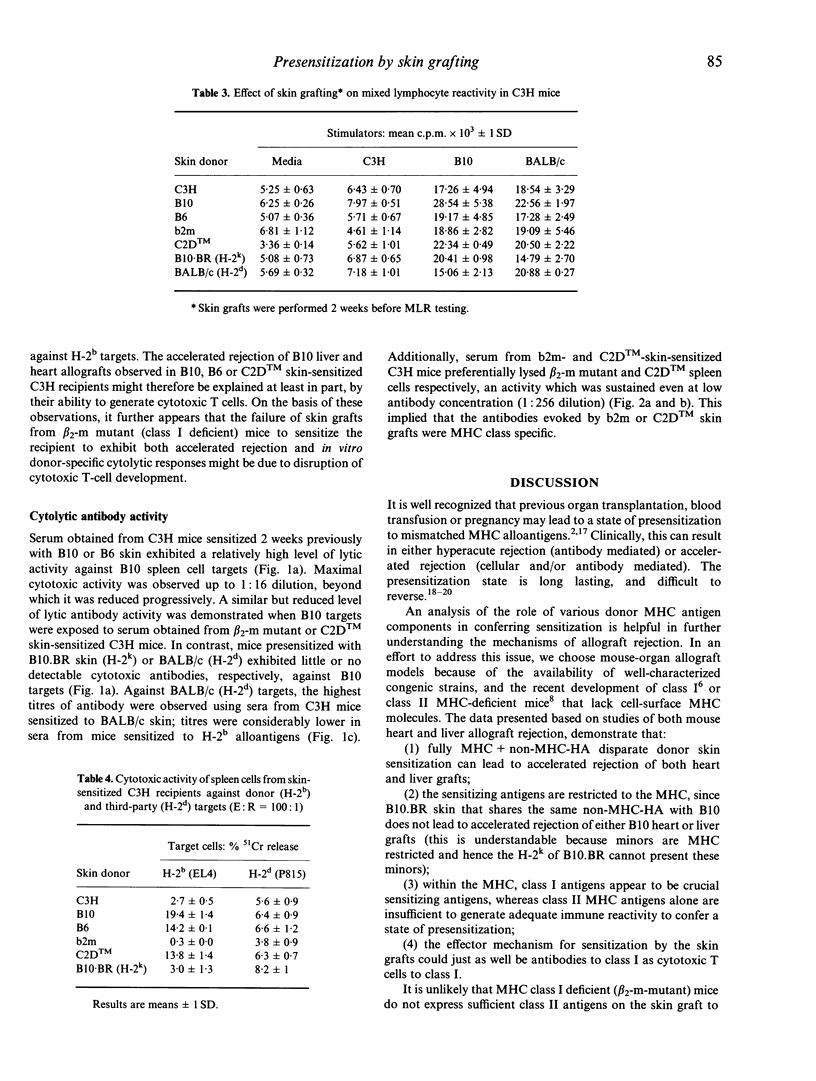
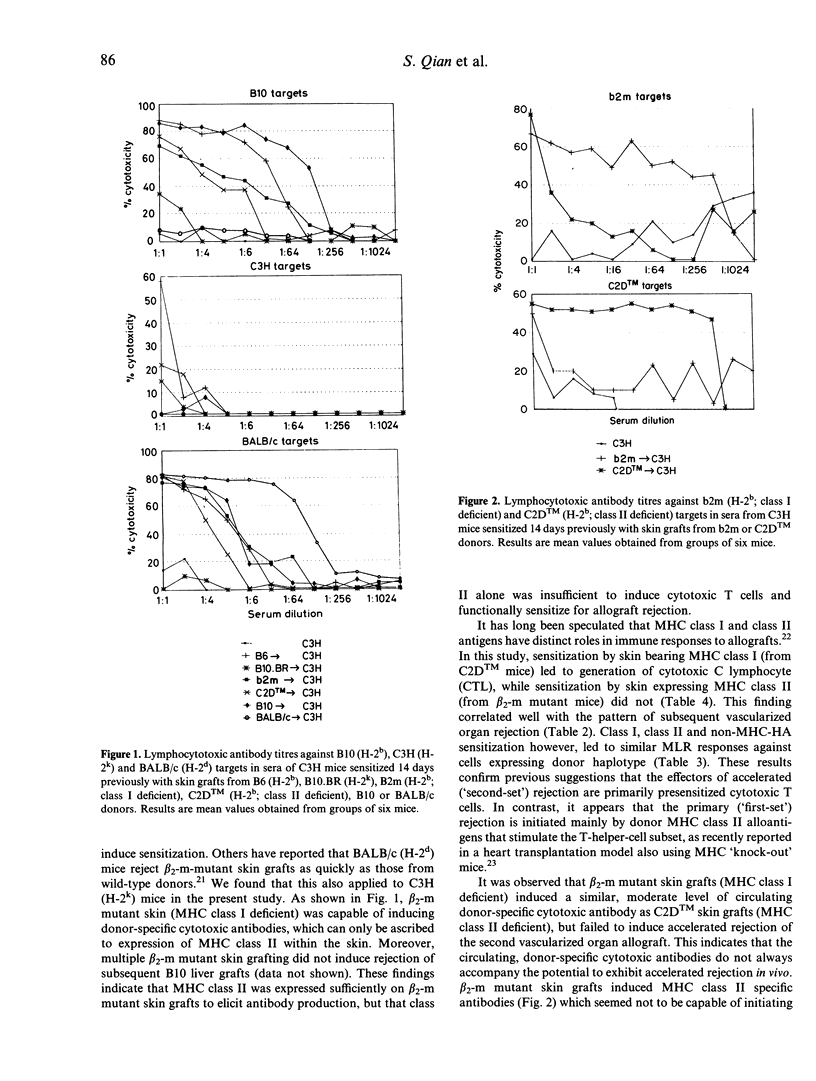
Livers but not hearts are accepted spontaneously without immunosuppression when transplanted from B10 (KbAbEbDb) to C3H (KkAkEkDk) mice. Both organs however, undergo accelerated rejection in C3H recipients presensitized with B10 skin grafts. In this study, we have investigated further the role of functional cell-surface major histocompatibility complex (MHC class I or class II molecules in allosensitization. Skin from transgenic MHC class I (b2mmlUncbcr; AbEb) or class II (C2DTM, KbDb) gene 'knockout' mice was grafted onto naive recipients 2-3 weeks prior to whole organ transplantation. When C3H hosts were presensitized with skin from C2DTM (class II deficient) mice, they promptly rejected (within 4 days) subsequently transplanted B10 liver or heart allografts. In contrast, presensitization with skin from b2m (beta 2-m mutant; class I deficient) mice did not significantly affect the survival of either organ graft. Maximal sensitization was established by day 14 after skin grafting and persisted for at least 12 weeks. Splenocytes obtained from C3H mice sensitized with skin from B10, B6 (KbAbEbDb), or C2DTM but not from b2m mice exhibited an H-2b-specific cytolytic response when tested in cell-mediated lymphocytotoxicity assays. Sera from C3H mice sensitized with B10 or b2m skin contained high titres of cytotoxic activity specifically against H-2b class I. Taken together, these observations suggest that in the strain combination studied, MHC class I rather than class II molecules play an important role in allosensitization. The results indicate the potential importance of avoiding transplantation of organs into recipients of secondary grafts from donors that share human leucocyte antigen (HLA) class I antigens with the first donor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Braun W. E. Laboratory and clinical management of the highly sensitized organ transplant recipient. Hum Immunol. 1989 Dec;26(4):245–260. doi: 10.1016/0198-8859(89)90003-7. [DOI] [PubMed] [Google Scholar]
- Cardell S., Merkenschlager M., Bodmer H., Chan S., Cosgrove D., Benoist C., Mathis D. The immune system of mice lacking conventional MHC class II molecules. Adv Immunol. 1994;55:423–440. doi: 10.1016/s0065-2776(08)60515-5. [DOI] [PubMed] [Google Scholar]
- Dahmen U., Qian S., Rao A. S., Demetris A. J., Fu F., Sun H., Gao L., Fung J. J., Starzl T. E. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. 1994 Jul 15;58(1):1–8. doi: 10.1097/00007890-199407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger R. B., Terasaki P. I., Opelz G., Malekzadeh M., Uittenbogaart C., Pennisi A. J., Fine R. Successful renal allografts across a positive cross-match for donor B-lymphocyte alloantigens. Lancet. 1976 Jul 10;2(7976):56–58. doi: 10.1016/s0140-6736(76)92282-0. [DOI] [PubMed] [Google Scholar]
- Goeken N. E. Differential stimulatory requirements and regulation of naive and primed human lymphocytes. Hum Immunol. 1984 Aug;10(4):251–263. doi: 10.1016/0198-8859(84)90090-9. [DOI] [PubMed] [Google Scholar]
- Goulmy E., Persijn G., Blokland E., D'Amaro J., van Rood J. J. Cell-mediated lympholysis studies in renal allograft recipients. Transplantation. 1981 Mar;31(3):210–217. doi: 10.1097/00007890-198103000-00014. [DOI] [PubMed] [Google Scholar]
- Grusby M. J., Johnson R. S., Papaioannou V. E., Glimcher L. H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991 Sep 20;253(5026):1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- Häyry P., von Willebrand E., Parthenais E., Nemlander A., Soots A., Lautenschlager I., Alfoldy P., Renkonen R. The inflammatory mechanisms of allograft rejection. Immunol Rev. 1984;77:85–142. doi: 10.1111/j.1600-065x.1984.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Lobo P. I., Wertervelt F. B., Jr, Rudolf L. E. Kidney transplantatability across a positive cross-match. Cross-match assays and distribution of B lymphocytes in donor tissues. Lancet. 1977 Apr 30;1(8018):925–928. doi: 10.1016/s0140-6736(77)92223-1. [DOI] [PubMed] [Google Scholar]
- Morris P. J., Ting A., Daar A. S., Oliver D. Letter: B-cell alloantibodies and renal allografts. Lancet. 1976 Aug 7;2(7980):312–313. doi: 10.1016/s0140-6736(76)90763-7. [DOI] [PubMed] [Google Scholar]
- Ono K., Lindsey E. S. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969 Feb;57(2):225–229. [PubMed] [Google Scholar]
- Qian S. G., Fung J. J., Demetris A. V., Ildstad S. T., Starzl T. E. Orthotopic liver transplantation in the mouse. Transplantation. 1991 Sep;52(3):562–564. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S., Demetris A. J., Murase N., Rao A. S., Fung J. J., Starzl T. E. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994 Apr;19(4):916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H. MHC class I-deficient mice. Adv Immunol. 1994;55:381–421. doi: 10.1016/s0065-2776(08)60514-3. [DOI] [PubMed] [Google Scholar]
- Sanfilippo F., Vaughn W. K., Bollinger R. R., Spees E. K. Comparative effects of pregnancy, transfusion, and prior graft rejection on sensitization and renal transplant results. Transplantation. 1982 Dec;34(6):360–366. doi: 10.1097/00007890-198212000-00010. [DOI] [PubMed] [Google Scholar]
- Streilein J. W., Strome P., Wood P. J. Failure of in vitro assays to predict accurately the existence of neonatally induced H-2 tolerance. Transplantation. 1989 Oct;48(4):630–634. [PubMed] [Google Scholar]
- Thomas J., Thomas F., Mendez-Picon G., Lee H. Immunological monitoring of long-surviving renal transplant recipients. Surgery. 1977 Feb;81(2):125–131. [PubMed] [Google Scholar]
- Thompson J. S., Bryne J. E., Hempel H. O., Oldfather J. W., Green W. F., Crowe D. O., Sanfilippo F., MacQueen J. M., McCalmon R. N., Tardiff G. N. Computer algorithm that predicts both acceptable and unacceptable private and public HLA class I antigens in highly sensitized patients. Transplant Proc. 1993 Feb;25(1 Pt 1):251–254. [PubMed] [Google Scholar]
- Ting A., Morris P. J. Development of donor-specific B lymphocyte antibodies after renal transplantation. No correlation with graft outcome. Transplantation. 1979 Jul;28(1):13–17. doi: 10.1097/00007890-197907000-00004. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Auchincloss H., Jr, Loring J. M., Chase C. M., Russell P. S., Jaenisch R. Skin graft rejection by beta 2-microglobulin-deficient mice. J Exp Med. 1992 Apr 1;175(4):885–893. doi: 10.1084/jem.175.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]


