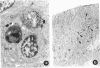
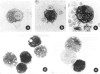
Abstract
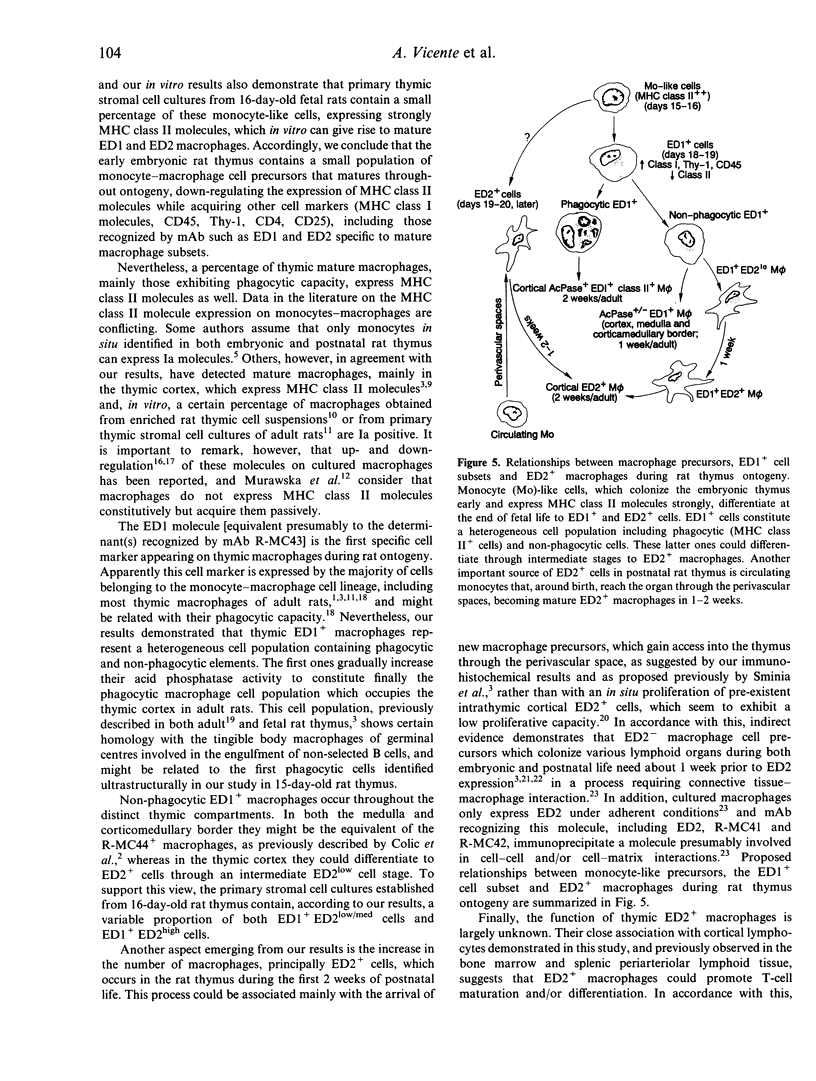
In the present study we combined electron microscopy, immunohistology and primary stromal cell cultures to analyse the ontogeny of rat thymic macrophages (M phi) in an attempt to clarify the relationships between the different macrophage cell subsets described in adult rat thymus. Although phagocytic cells were observed in 15-day-old fetal thymus, monoclonal antibodies (mAb) which recognize different adult macrophage types were unable to identify positive cells until the end of embryonic life. However, our in vitro results from primary thymic stromal cell cultures of 16-day-old fetal rats, and the phenotyping of enriched thymic CD2- cell suspensions, demonstrated that monocyte-like cells which strongly expressed major histocompatibility complex (MHC) class II molecules colonized the embryonic thymus early, giving rise later to distinct macrophage subsets. During the process of maturation, macrophage precursors gradually lost their MHC class II expression, acquired other surface markers (CD45, Thy-1, CD25, CD4, etc.) and increased the acid phosphatase activity. In this respect, ED1+ macrophages, which appeared for the first time in the last stages of embryonic life, consisted of a MHC class II molecule-expressing phagocytic cell population, presumably involved in the elimination of non-selected cortical thymocytes, and of non-phagocytic cells which, in the thymic cortex, might differentiate to ED2+ macrophages throughout ED1+ED2lo/med and ED1+ ED2high intermediate cell stages, observed in vitro in 16-day-old fetal thymic stromal cell cultures. At the end of embryonic life and during the postnatal period the numbers of thymic macrophages increased, particularly in the medulla and corticomedullary border (CMZ), and more slowly in the thymic cortex. This increase was presumably due to the arrival, through perivascular spaces, of new macrophage progenitors, rather than in situ proliferation of pre-existent mature macrophages. The possible function of different thymic macrophage subsets, as well as the relationships between themselves and with their presumptive monocyte-like precursors, are discussed.
Full text
PDF

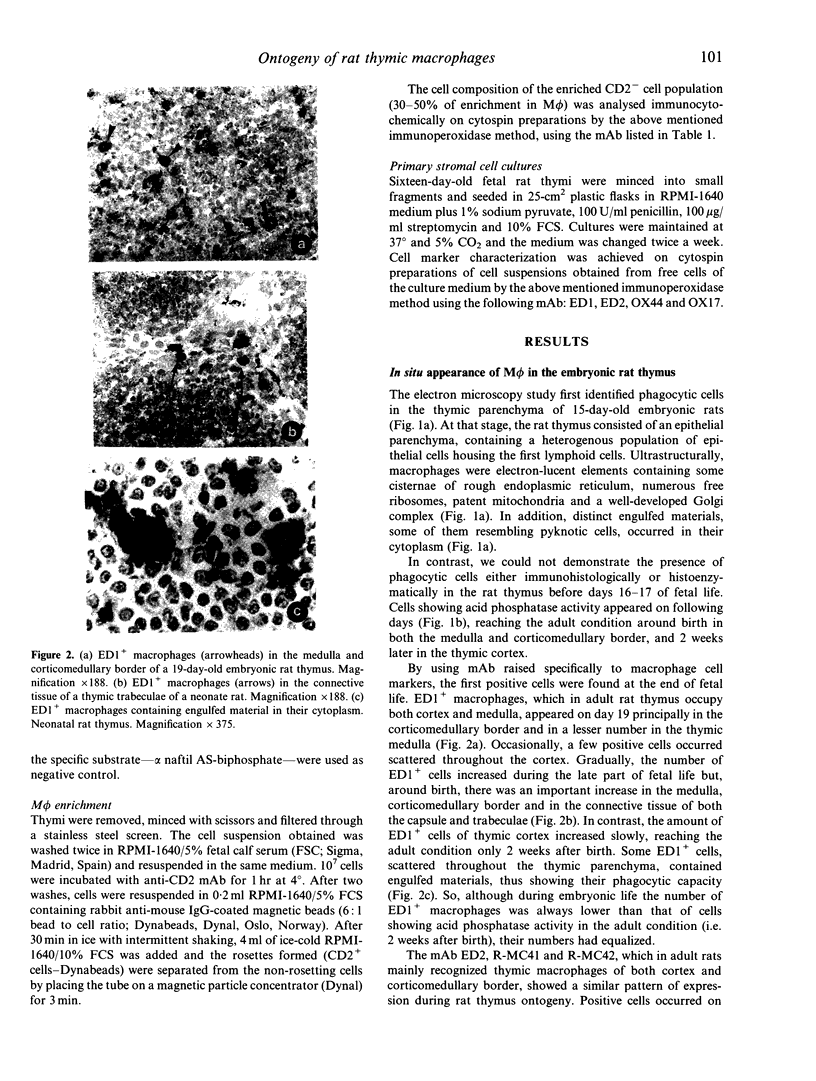
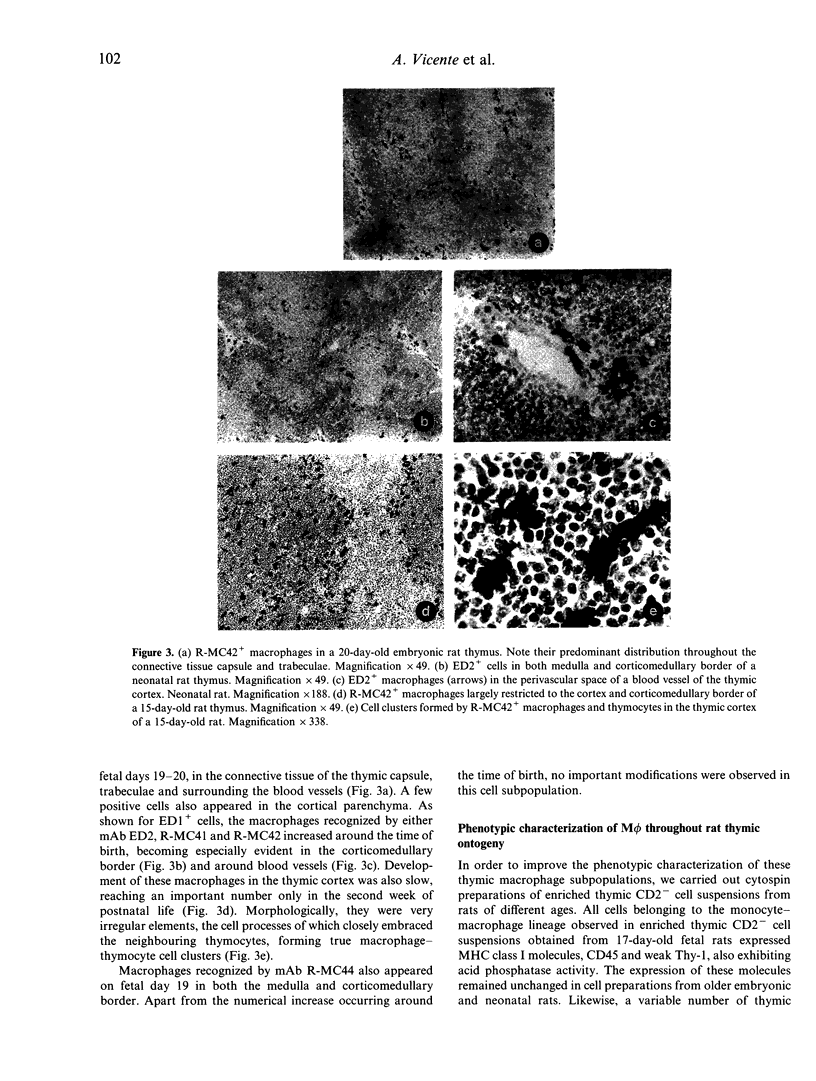


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colić M., Drabek D. Phenotype and adhesion characteristics of rat thymic macrophages cultivated in serum-free medium. Thymus. 1993 Feb;21(1):43–60. [PubMed] [Google Scholar]
- Colić M., Popović L. J., Gasić S., Dragojević-Simić V., Milićević N. M., Matanović D., Dujić A. Immunohistochemical characterization of rat thymic non-lymphoid cells. II. Macrophages and granulocytes defined by monoclonal antibodies. Immunology. 1990 Mar;69(3):416–422. [PMC free article] [PubMed] [Google Scholar]
- Colić M., Popović L., Gasić S., Drabek D., Dujić A. Primary culture of rat thymic non-lymphoid cells: influence of culture time on the expression of macrophage differentiation antigens defined by monoclonal antibodies. Thymus. 1991 Dec;18(4):243–256. [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn A. M., Schutte R., Köhler Y. G., Korn C., Hoefsmit E. C. Characterization of the population of phagocytic cells in thymic cell suspensions. A morphological and cytochemical study. Cell Tissue Res. 1983;231(2):313–323. doi: 10.1007/BF00222183. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Sminia T., Köhler Y. G., Janse E. M., Hoefsmit E. C. Ontogeny of the rat thymus micro-environment: development of the interdigitating cell and macrophage populations. Dev Comp Immunol. 1984 Fall;8(4):947–956. doi: 10.1016/0145-305x(84)90077-6. [DOI] [PubMed] [Google Scholar]
- Hsiao L., Takahashi K., Takeya M., Arao T. Differentiation and maturation of macrophages into interdigitating cells and their multicellular complex formation in the fetal and postnatal rat thymus. Thymus. 1991 Jun;17(4):219–235. [PubMed] [Google Scholar]
- Murawska M. B., Duijvestijn A. M., Klatter F. A., Ammerlaan W., Meedendorp B., Nieuwenhuis P. Differential kinetics of various subsets of thymic bone marrow-derived stromal cells in rat chimeras. Scand J Immunol. 1991 Apr;33(4):473–484. doi: 10.1111/j.1365-3083.1991.tb01796.x. [DOI] [PubMed] [Google Scholar]
- Nabarra B., Papiernik M. Phenotype of thymic stromal cells. An immunoelectron microscopic study with anti-IA, anti-MAC-1, and anti-MAC-2 antibodies. Lab Invest. 1988 May;58(5):524–531. [PubMed] [Google Scholar]
- Navarro R., Ardavin C., Fontecha A. M., Alvarez A., Zapata A. In vitro characterization of rat thymic macrophages. Immunology. 1991 May;73(1):114–116. [PMC free article] [PubMed] [Google Scholar]
- Papiernik M., Dombret H., Stefanos S., Wietzerbin J. Control of Ia antigen expression on phagocytic cells of the thymic reticulum by interferon-gamma and prostaglandins. Eur J Immunol. 1986 Mar;16(3):296–300. doi: 10.1002/eji.1830160316. [DOI] [PubMed] [Google Scholar]
- Robinson J. H. The ontogeny of antigen-presenting cells in fetal thymus evaluated by MLR stimulation. J Immunol. 1983 Apr;130(4):1592–1595. [PubMed] [Google Scholar]
- Robinson J. H. The ontogeny of thymic macrophages: thymic macrophages express Ia from 15 days gestation onwards in the mouse. Cell Immunol. 1984 Apr 1;84(2):422–426. doi: 10.1016/0008-8749(84)90115-1. [DOI] [PubMed] [Google Scholar]
- Sminia T., van Asselt A. A., van de Ende M. B., Dijkstra C. D. Rat thymus macrophages: an immunohistochemical study on fetal, neonatal and adult thymus. Thymus. 1986;8(3):141–150. [PubMed] [Google Scholar]
- Westermann J., Ronneberg S., Fritz F. J., Pabst R. Proliferation of macrophage subpopulations in the adult rat: comparison of various lymphoid organs. J Leukoc Biol. 1989 Sep;46(3):263–269. doi: 10.1002/jlb.46.3.263. [DOI] [PubMed] [Google Scholar]
- Zepp F., Schulte-Wissermann H., Mannhardt W. Macrophage subpopulations regulate intrathymic T-cell development. I: Ia-positive macrophages augment thymocyte proliferation. Thymus. 1984;6(5):279–293. [PubMed] [Google Scholar]
- van Rees E. P., Dijkstra C. D. Postnatal development of non-lymphoid and lymphoid cell populations in situ in diabetes-prone BB rats. Adv Exp Med Biol. 1988;237:737–743. doi: 10.1007/978-1-4684-5535-9_110. [DOI] [PubMed] [Google Scholar]
- van Rees E. P., Döpp E. A., Dijkstra C. D., Sminia T. The postnatal development of cell populations in the rat popliteal lymph node. An immunohistochemical study. Cell Tissue Res. 1985;242(2):391–398. doi: 10.1007/BF00214553. [DOI] [PubMed] [Google Scholar]