Abstract
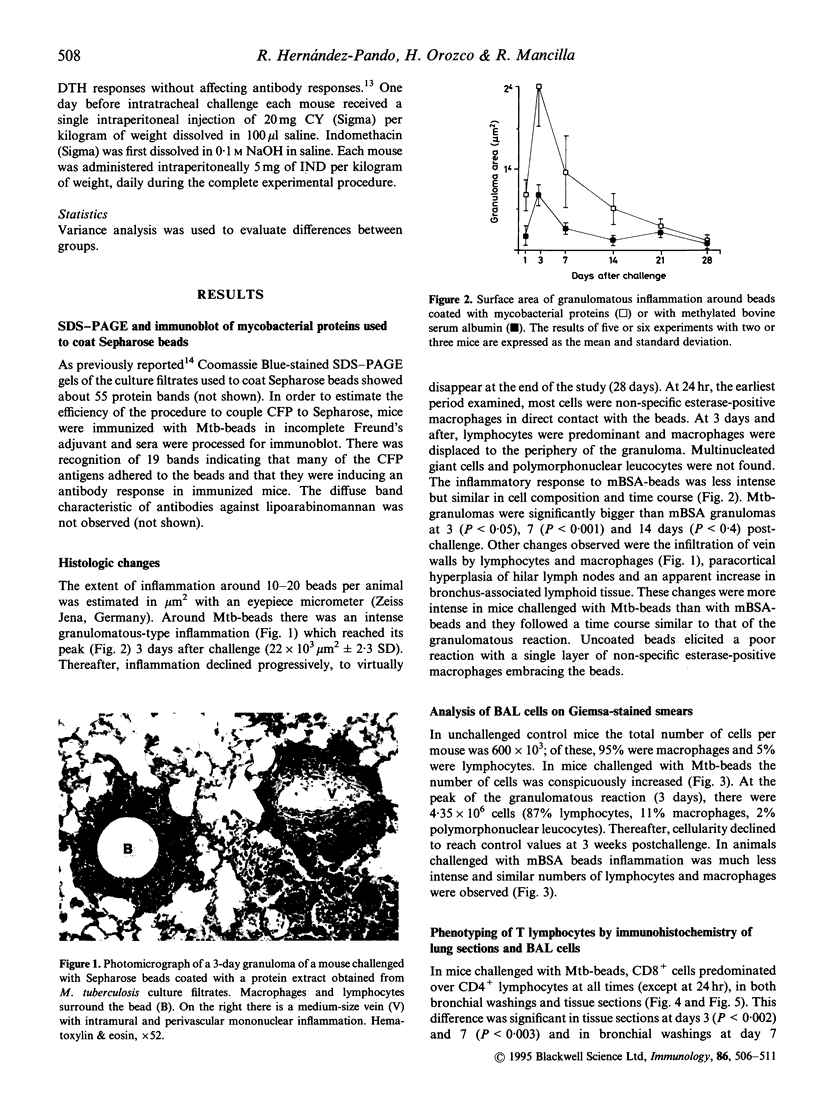
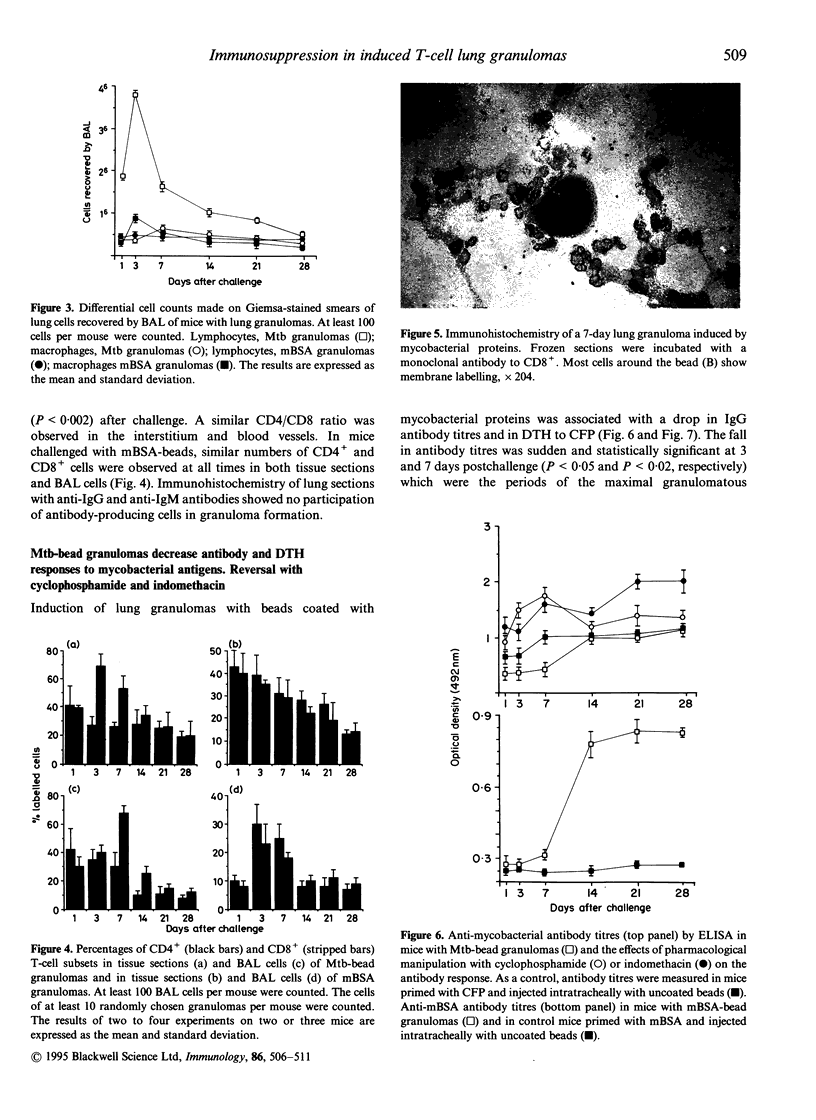
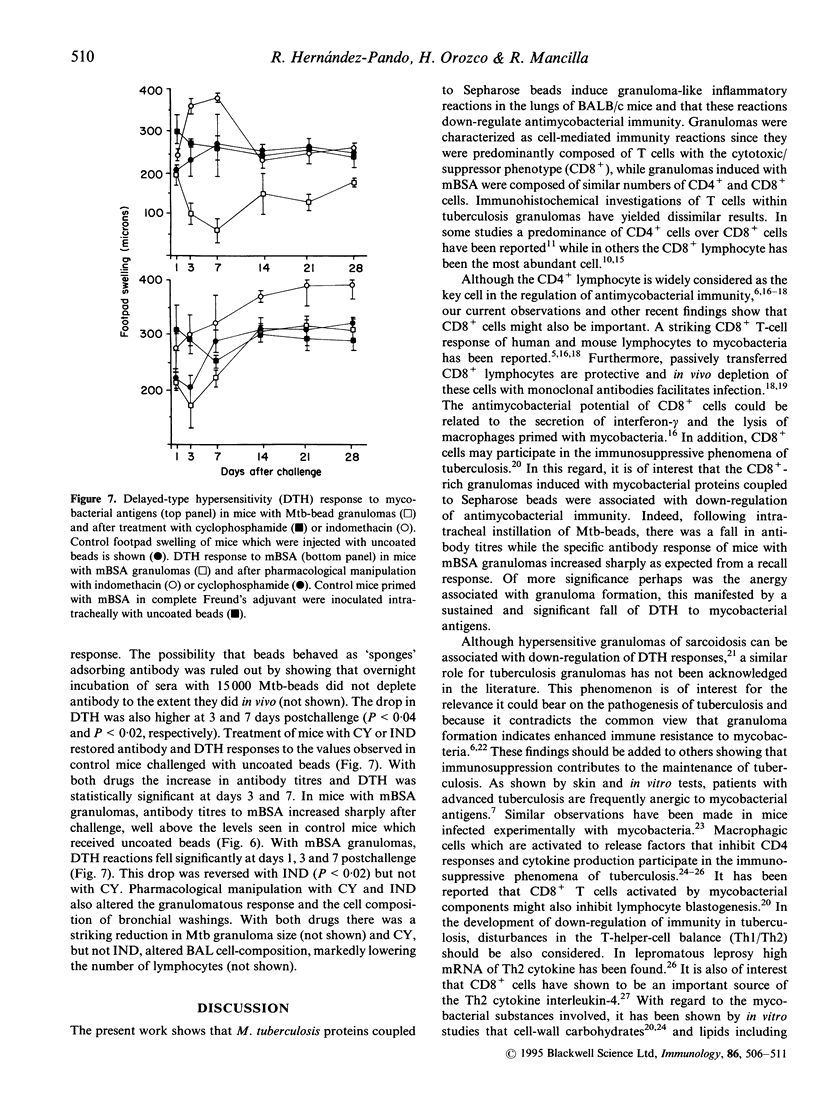
We induced lung granulomas in BALB/c mice by intratracheal instillation of Sepharose beads coated with a Mycobacterium tuberculosis protein extract. Granulomas composed of macrophages and lymphocytes were induced. The granulomatous reaction reached its peak 3-7 days after challenge and lasted for approximately 1 month. Immunolabelling of tissue sections and bronchial washings revealed that granulomas were predominantly composed of T lymphocytes with the cytotoxic-suppressor phenotype (CD8+). Granulomas were associated with a significant decrease in anti-mycobacterial immunity manifested by a drop in delayed-type hypersensitivity reactions and antibody titres. The immunosuppressive phenomena were abolished with cyclophosphamide or indomethacin. Control granulomas induced with methylated bovine serum albumin (BSA) were smaller and composed by similar numbers of CD4+ and CD8+ cells. BSA granulomas did not alter antibody titres but they decreased delayed-type hypersensitivity to BSA which was restored to normal with indomethacin but not with cyclophosphamide. Our findings show that mycobacterial proteins anchored to Sepharose beads are granulomatogenic and that they preferentially recruit CD8+ cells which, together with locally produced prostaglandins, down-modulate cell-mediated and humoral immunity to mycobacterial antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W., Hayden B. J., Gershon R. K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975 Mar 1;141(3):697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., de Sousa J. P., Davis T. L., Wright E. L., Bachelet M., Rastogi N. Immunomodulation of human peripheral blood mononuclear cell functions by defined lipid fractions of Mycobacterium avium. Infect Immun. 1993 Dec;61(12):5286–5293. doi: 10.1128/iai.61.12.5286-5293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Murray C. J. Tuberculosis: commentary on a reemergent killer. Science. 1992 Aug 21;257(5073):1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Watson S. R. Suppressor T-cells in BCG-infected mice. Infect Immun. 1979 Aug;25(2):491–496. doi: 10.1128/iai.25.2.491-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Espitia C., Cervera I., González R., Mancilla R. A 38-kD Mycobacterium tuberculosis antigen associated with infection. Its isolation and serologic evaluation. Clin Exp Immunol. 1989 Sep;77(3):373–377. [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Holt P. G. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986 Feb;63(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Crystal R. G. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981 Aug 20;305(8):429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- Kato K., Yamamoto K. Involvement of prostaglandin E1 in delayed-type hypersensitivity suppression induced with live Mycobacterium bovis BCG. Infect Immun. 1982 Apr;36(1):426–429. doi: 10.1128/iai.36.1.426-429.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- Muscoplat C. C., Rakich P. M., Thoen C. O., Johnson D. W. Enhancement of lymphocyte blastogenic and delayed hypersensitivity skin responses by indomethacin. Infect Immun. 1978 Jun;20(3):627–631. doi: 10.1128/iai.20.3.627-631.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Andersen P., Boom W. H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993 Jun;167(6):1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992 Jan 1;148(1):189–196. [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Randhawa P. S. Lymphocyte subsets in granulomas of human tuberculosis: an in situ immunofluorescence study using monoclonal antibodies. Pathology. 1990 Jul;22(3):153–155. doi: 10.3109/00313029009063555. [DOI] [PubMed] [Google Scholar]
- Roch F., Bach M. A. Strain differences in mouse cellular responses to Mycobacterium lepraemurium and BCG subcutaneous infections. I. Analysis of cell surface phenotype in local granulomas. Clin Exp Immunol. 1990 Jun;80(3):332–338. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. Progress in the immunology of the mycobacterioses. Clin Exp Immunol. 1987 Jul;69(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A. Mycobacteria and autoimmunity. Immunol Today. 1988 Jun;9(6):178–182. doi: 10.1016/0167-5699(88)91294-7. [DOI] [PubMed] [Google Scholar]
- Sussman G., Wadee A. A. Production of a suppressor factor by CD8+ lymphocytes activated by mycobacterial components. Infect Immun. 1991 Aug;59(8):2828–2835. doi: 10.1128/iai.59.8.2828-2835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toossi Z., Kleinhenz M. E., Ellner J. J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986 May 1;163(5):1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- van den Oprd J. J., de Wolf-Peeters C., Facchetti F., Desmet V. J. Cellular composition of hypersensitivity-type granulomas: immunohistochemical analysis of tuberculous and sarcoidal lymphadenitis. Hum Pathol. 1984 Jun;15(6):559–565. doi: 10.1016/s0046-8177(84)80010-6. [DOI] [PubMed] [Google Scholar]




