Abstract
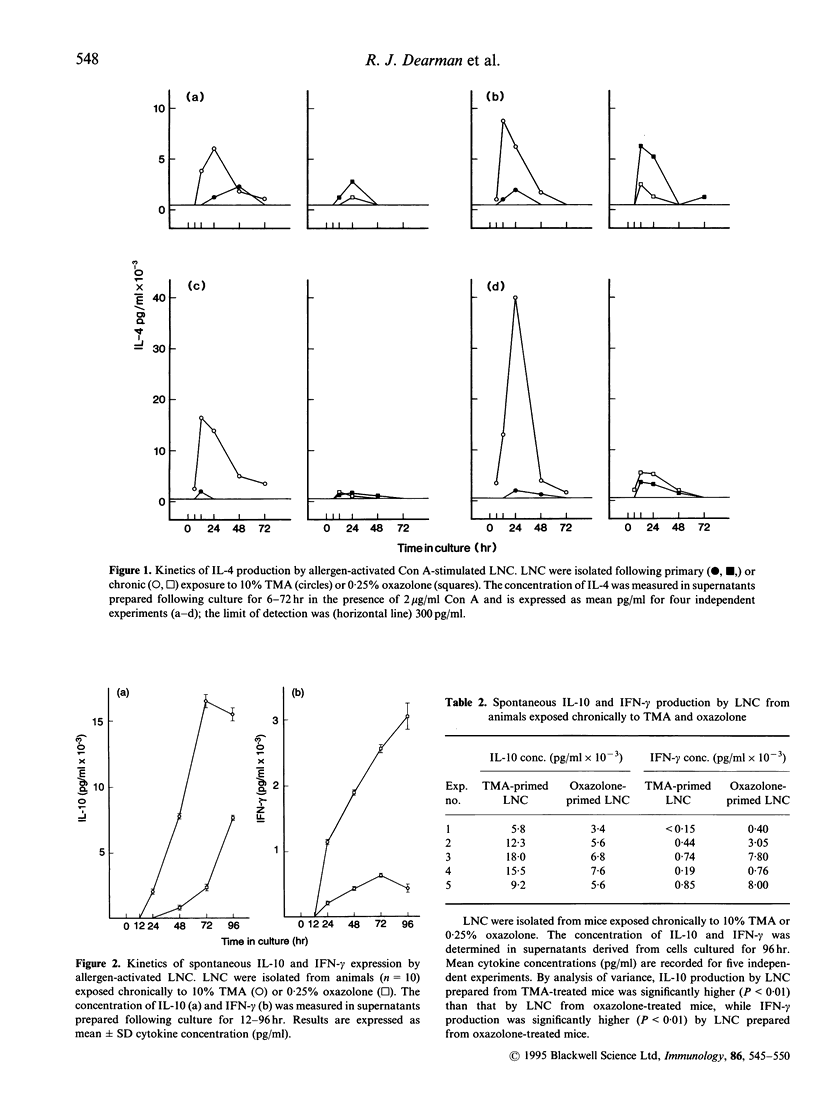
It has been demonstrated previously that a selective pattern of mitogen-inducible interleukin-4 (IL-4) production becomes apparent in mice after temporal evolution of the immune response to different classes of chemical allergen. Mitogen-stimulated draining lymph node cells (LNC) isolated after primary exposure to both the respiratory allergen trimellitic anhydride (TMA) and oxazolone, a contact allergen, secreted similar amounts of IL-4. Following secondary exposure, however, TMA, but not oxazolone, caused a marked increase in IL-4 production, consistent with the stimulation by TMA of a T-helper type-2 (Th2)-type response. In the present study, cytokine production characteristic of Th1 (interferon-gamma; IFN-gamma) and Th2 (IL-4 and IL-10) cell activation was examined following chronic exposure of mice to allergen over a 13-day period. In accord with previous studies, chronic exposure to TMA, but not to oxazolone, resulted in a substantial potentiation of mitogen-inducible IL-4 secretion. In addition, spontaneous IL-10 production by TMA-activated LNC was significantly higher than by cells prepared from oxazolone-exposed animals. The lower levels of Il-4 and IL-10 elaborated by oxazolone-activated LNC were not attributable to a reduced potential to secrete cytokine per se, as significantly more IFN-gamma was produced compared with TMA-activated LNC. It is proposed that these divergent cytokine production patterns reflect the selective stimulation of Th1- and Th2-type responses by contact and respiratory chemical allergens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abehsira-Amar O., Gibert M., Joliy M., Thèze J., Jankovic D. L. IL-4 plays a dominant role in the differential development of Tho into Th1 and Th2 cells. J Immunol. 1992 Jun 15;148(12):3820–3829. [PubMed] [Google Scholar]
- Alexander J., Snoke K., Ruppert J., Sidney J., Wall M., Southwood S., Oseroff C., Arrhenius T., Gaeta F. C., Colón S. M. Functional consequences of engagement of the T cell receptor by low affinity ligands. J Immunol. 1993 Jan 1;150(1):1–7. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Croft M., Carter L., Swain S. L., Dutton R. W. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994 Nov 1;180(5):1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A., Aste-Amezaga M., Valiante N. M., Ma X., Kubin M., Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993 Sep 1;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearman R. J., Kimber I. Differential stimulation of immune function by respiratory and contact chemical allergens. Immunology. 1991 Apr;72(4):563–570. [PMC free article] [PubMed] [Google Scholar]
- Dearman R. J., Kimber I. Divergent immune responses to respiratory and contact chemical allergens: antibody elicited by phthalic anhydride and oxazolone. Clin Exp Allergy. 1992 Feb;22(2):241–250. doi: 10.1111/j.1365-2222.1992.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Dearman R. J., Ramdin L. S., Basketter D. A., Kimber I. Inducible interleukin-4-secreting cells provoked in mice during chemical sensitization. Immunology. 1994 Apr;81(4):551–557. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Holmes J., Ohara J., Tung A. S., Sample J. V., Paul W. E. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988 Oct 1;141(7):2335–2341. [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Urban J. F., Jr, Snapper C. M., Ohara J., Paul W. E. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9675–9678. doi: 10.1073/pnas.83.24.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989 Nov 1;143(9):2887–2893. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Kamogawa Y., Minasi L. A., Carding S. R., Bottomly K., Flavell R. A. The relationship of IL-4- and IFN gamma-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993 Dec 3;75(5):985–995. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- Kimber I., Pierce B. B., Mitchell J. A., Kinnaird A. Depression of lymph node cell proliferation induced by oxazolone. Int Arch Allergy Appl Immunol. 1987;84(3):256–262. doi: 10.1159/000234432. [DOI] [PubMed] [Google Scholar]
- Maguire H. C., Jr Murine recombinant interleukin-12 increases the acquisition of allergic contact dermatitis in the mouse. Int Arch Allergy Immunol. 1995 Feb;106(2):166–168. doi: 10.1159/000236839. [DOI] [PubMed] [Google Scholar]
- McKnight A. J., Zimmer G. J., Fogelman I., Wolf S. F., Abbas A. K. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994 Mar 1;152(5):2172–2179. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R. Regulation of immune responses by T cells with different cytokine secretion phenotypes: role of a new cytokine, cytokine synthesis inhibitory factor (IL10). Int Arch Allergy Appl Immunol. 1991;94(1-4):110–115. doi: 10.1159/000235340. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Boulay J. L., Finkelman F., Barbier S., Ben-Sasson S. Z., Le Gros G., Paul W. E. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992 Mar 15;148(6):1652–1656. [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Regulation of IgG1 and IgE production by interleukin 4. Immunol Rev. 1988 Feb;102:51–75. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Swain S. L., Bradley L. M., Croft M., Tonkonogy S., Atkins G., Weinberg A. D., Duncan D. D., Hedrick S. M., Dutton R. W., Huston G. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991 Oct;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990 Dec 1;145(11):3796–3806. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993 Jul;14(7):335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]


