Abstract
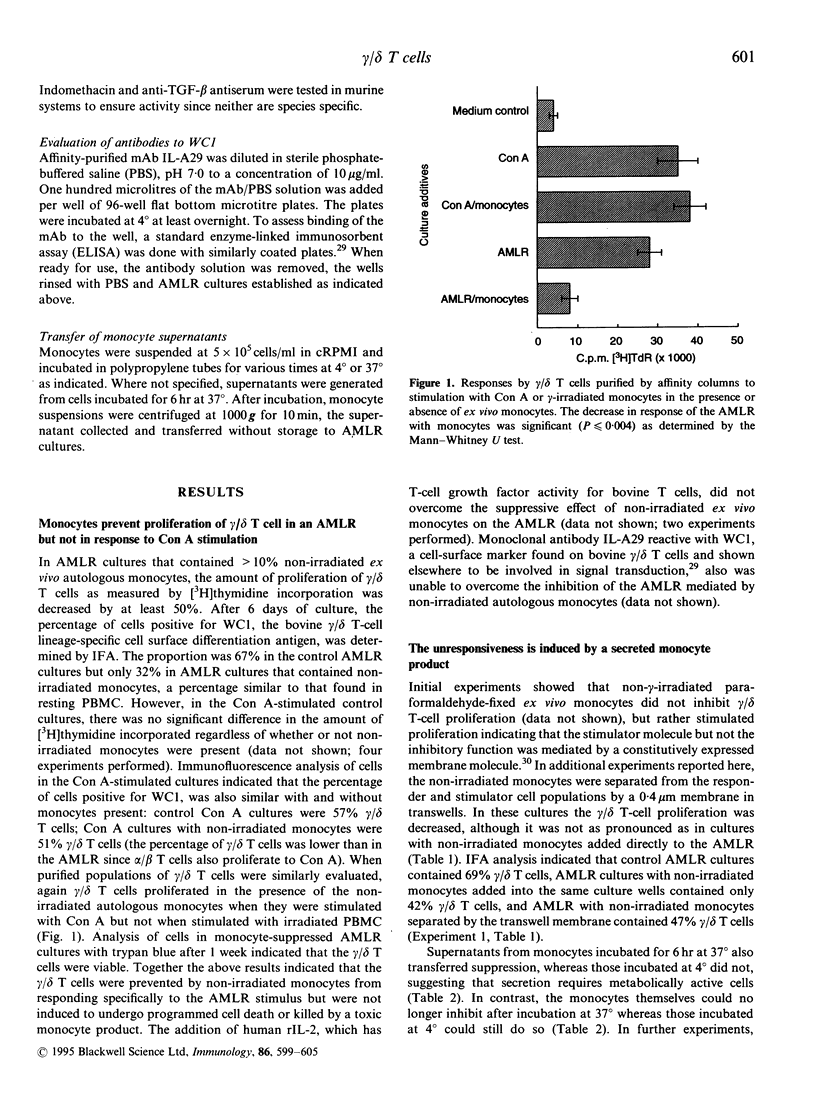
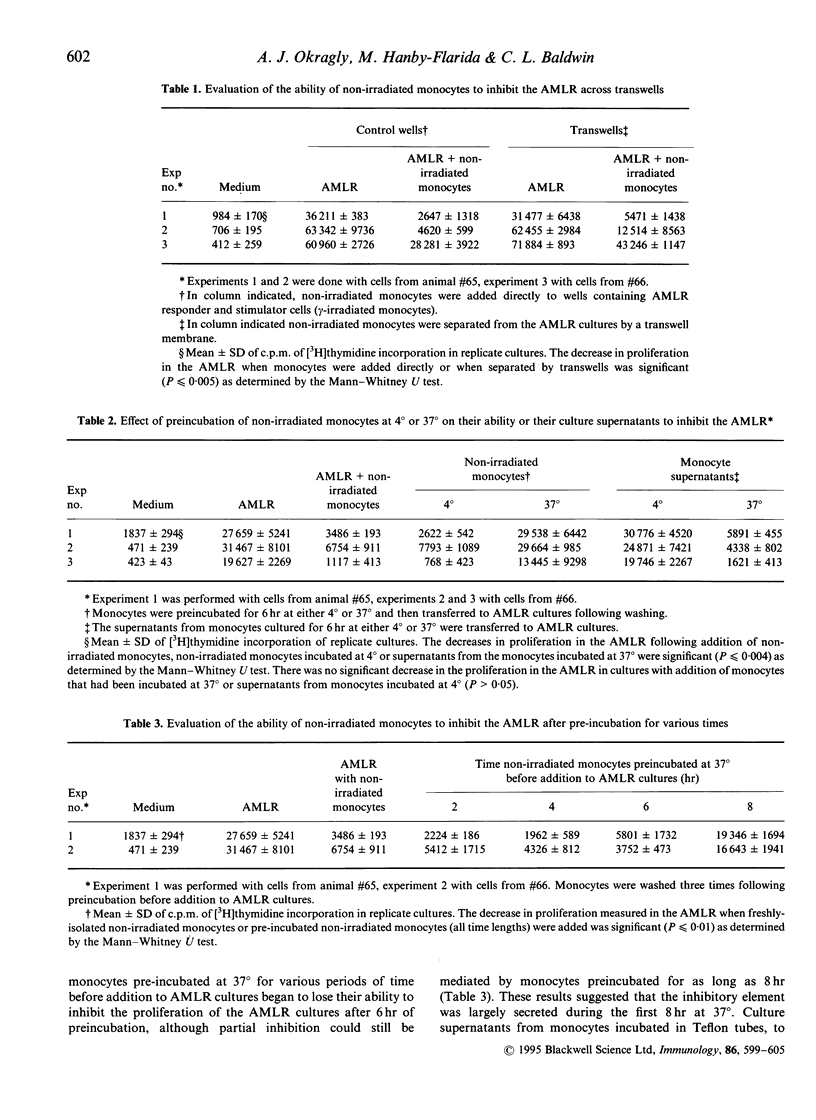
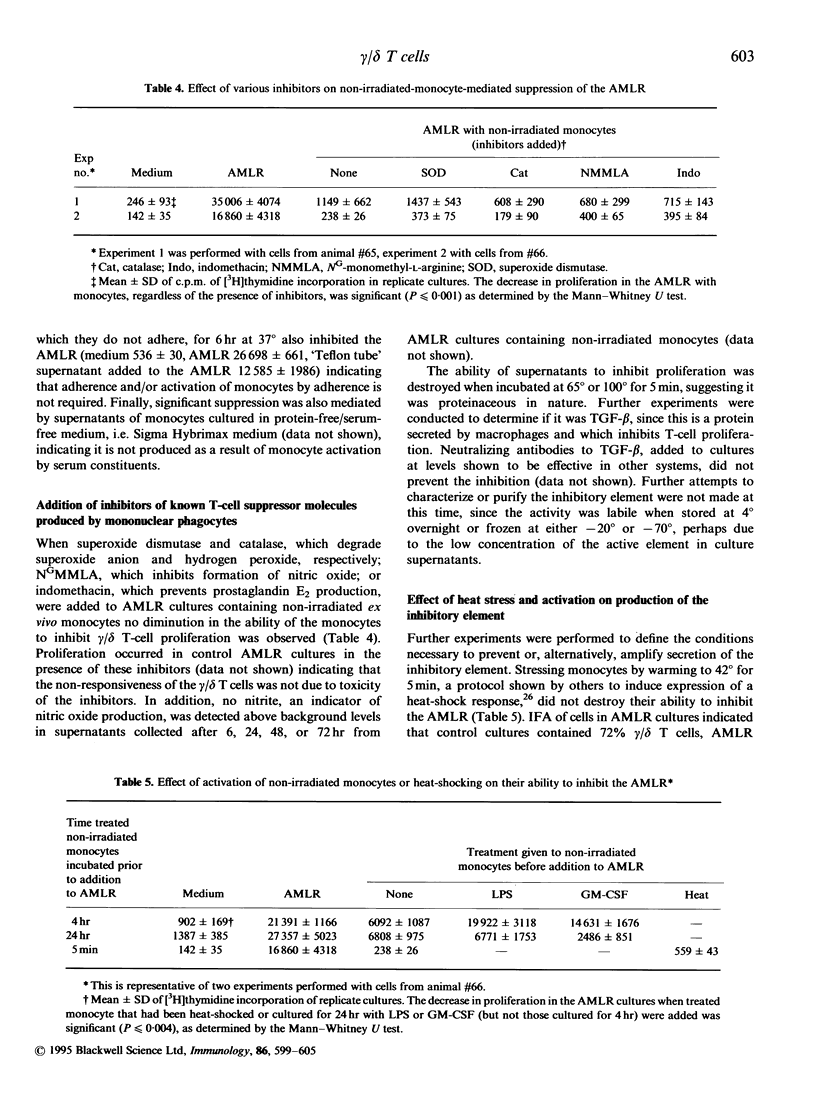
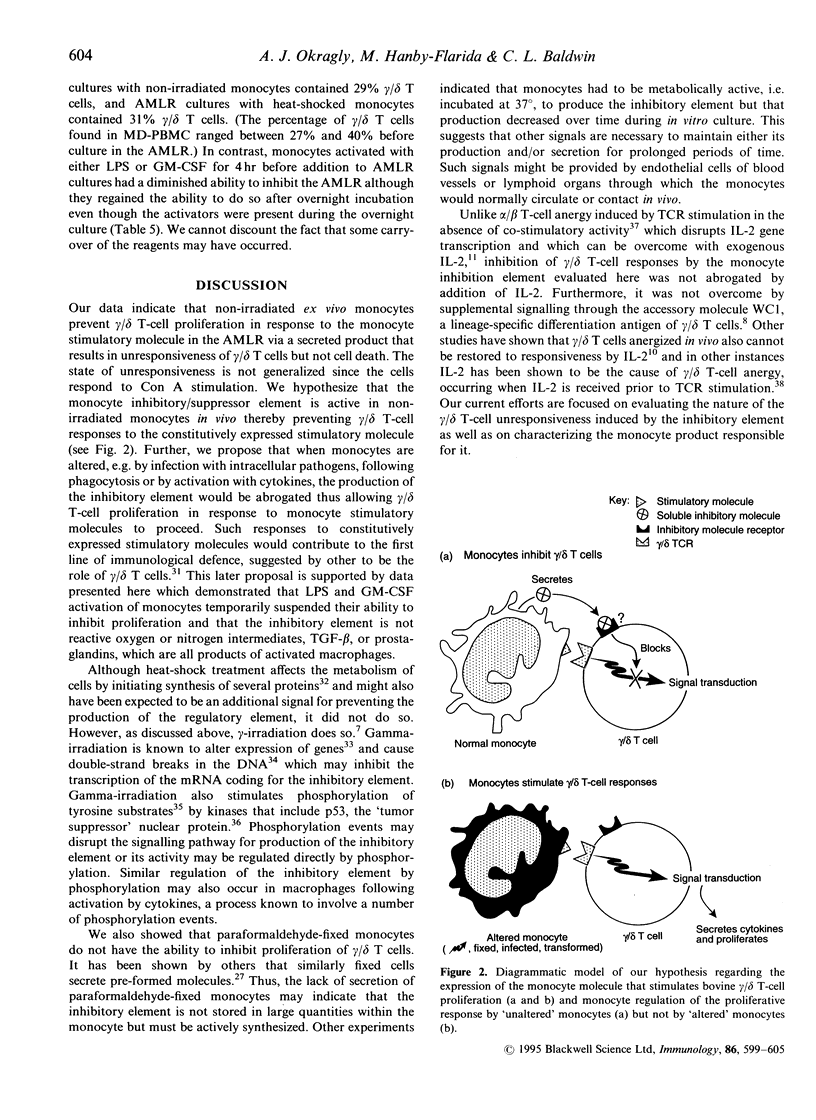
Gamma-irradiated ex vivo bovine monocytes induce proliferation of gamma/delta T cells in the autologous mixed lymphocyte reaction (AMLR), whereas when not irradiated they prevent this response. In contrast, non-irradiated autologous monocytes have no effect on bovine alpha/beta T-cell proliferation in the allogenic MLR suggesting that the regulation is specific for gamma/delta T-cell responses. Here, we showed that the inhibition was not mediated by inducing cell death and that the ability of ex vivo monocytes to prevent proliferation of gamma/delta T cells was not generalized in that gamma/delta T cells still responded to mitogenic stimulation. Inhibition of the AMLR by non-irradiated monocytes could not be overcome by addition of interleukin-2 to the cultures or by costimulation with antibodies to WC1, a gamma/delta T-cell-specific cell-surface differentiation antigen shown elsewhere by us to be involved in activation of gamma/delta T cells. Furthermore, we showed that monocytes inhibited gamma/delta T-cell responses via a soluble product since inhibition occurred even when monocytes and gamma/delta T cells were separated by membranes of transwells or when supernatants from monocyte cultures were added to AMLR cultures. Maximal secretion of the inhibitory product by the monocytes occurred during the first 6 hr of in vitro culture at 37 degrees, rapidly decreased thereafter, and did not occur when monocytes were incubated at 4 degrees. The inhibition was not attributable to nitric oxide, reactive oxygen intermediates, prostaglandin E2 or transforming growth factor-beta (TGF-beta) but the ability of monocyte supernatants to mediate inhibition was sensitive to heating at 65 degrees. Lipopolysaccharide and granulocyte-macrophage colony-stimulating factor activation of monocytes temporarily abrogated their ability to inhibit proliferation. In contrast, heat-shocking had no effect on their ability to inhibit. We hypothesize that non-irradiated monocytes produce the inhibitory material in vivo in order to regulate gamma/delta T-cell responses to self-derived monocyte membrane components, but that when monocytes are altered by infection, transformation, irradiation, or cytokine activation, production of the inhibitor is temporarily suspended allowing stimulation of gamma/delta T cells to occur.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. L., Teale A. J., Naessens J. G., Goddeeris B. M., MacHugh N. D., Morrison W. I. Characterization of a subset of bovine T lymphocytes that express BoT4 by monoclonal antibodies and function: similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986 Jun 15;136(12):4385–4391. [PubMed] [Google Scholar]
- Barrett T. A., Delvy M. L., Kennedy D. M., Lefrancois L., Matis L. A., Dent A. L., Hedrick S. M., Bluestone J. A. Mechanism of self-tolerance of gamma/delta T cells in epithelial tissue. J Exp Med. 1992 Jan 1;175(1):65–70. doi: 10.1084/jem.175.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born W. K., Harshan K., Modlin R. L., O'Brien R. L. The role of gamma delta T lymphocytes in infection. Curr Opin Immunol. 1991 Aug;3(4):455–459. doi: 10.1016/0952-7915(91)90002-i. [DOI] [PubMed] [Google Scholar]
- Clevers H., MacHugh N. D., Bensaid A., Dunlap S., Baldwin C. L., Kaushal A., Iams K., Howard C. J., Morrison W. I. Identification of a bovine surface antigen uniquely expressed on CD4-CD8- T cell receptor gamma/delta+ T lymphocytes. Eur J Immunol. 1990 Apr;20(4):809–817. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- Eichhorn M., Prospero T. D., Heussler V. T., Dobbelaere D. A. Antibodies against major histocompatibility complex class II antigens directly inhibit the growth of T cells infected with Theileria parva without affecting their state of activation. J Exp Med. 1993 Sep 1;178(3):769–776. doi: 10.1084/jem.178.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. A., Baldwin C. L., MacHugh N. D., Bensaid A., Teale A. J., Goddeeris B. M., Morrison W. I. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986 Jul;58(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Ferrick D. A., Gemmell-Hori L. Potential developmental role for self-reactive T cells bearing gamma-delta T cell receptors specific for heat-shock proteins. Chem Immunol. 1992;53:17–31. [PubMed] [Google Scholar]
- Goddeeris B. M., Baldwin C. L., ole-MoiYoi O., Morrison W. I. Improved methods for purification and depletion of monocytes from bovine peripheral blood mononuclear cells. Functional evaluation of monocytes in responses to lectins. J Immunol Methods. 1986 May 22;89(2):165–173. doi: 10.1016/0022-1759(86)90354-6. [DOI] [PubMed] [Google Scholar]
- Goddeeris B. M., Morrison W. I., Naessens J., Magondu J. G. The bovine autologous mixed leukocyte reaction: a proliferative response of non-T cells under the control of monocytes. Immunobiology. 1987 Dec;176(1-2):47–62. doi: 10.1016/S0171-2985(87)80099-2. [DOI] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Havran W. L., Chien Y. H., Allison J. P. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science. 1991 Jun 7;252(5011):1430–1432. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- Jadus M. R., Parkman R. The selective growth of murine newborn-derived suppressor cells and their probable mode of action. J Immunol. 1986 Feb 1;136(3):783–792. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Janssen O., Wesselborg S., Heckl-Ostreicher B., Pechhold K., Bender A., Schondelmaier S., Moldenhauer G., Kabelitz D. T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor gamma delta + T cells. J Immunol. 1991 Jan 1;146(1):35–39. [PubMed] [Google Scholar]
- Jenkins M. K. The role of cell division in the induction of clonal anergy. Immunol Today. 1992 Feb;13(2):69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massagué J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science. 1993 Apr 23;260(5107):536–539. doi: 10.1126/science.8475385. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991 Oct 31;353(6347):858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Mellouk S., Green S. J., Nacy C. A., Hoffman S. L. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991 Jun 1;146(11):3971–3976. [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Miller J. F. The discovery of the immunological function of the thymus. Immunol Today. 1991 Jan;12(1):42–45. doi: 10.1016/0167-5699(91)90111-6. [DOI] [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Newell M. K., Haughn L. J., Maroun C. R., Julius M. H. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990 Sep 20;347(6290):286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Spaner D., Migita K., Ochi A., Shannon J., Miller R. G., Pereira P., Tonegawa S., Phillips R. A. Gamma delta T cells differentiate into a functional but nonproliferative state during a normal immune response. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8415–8419. doi: 10.1073/pnas.90.18.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttles J., Carruth L. M., Mizel S. B. Detection of IL-1 alpha and IL-1 beta in the supernatants of paraformaldehyde-treated human monocytes. Evidence against a membrane form of IL-1. J Immunol. 1990 Jan 1;144(1):170–174. [PubMed] [Google Scholar]
- Uckun F. M., Tuel-Ahlgren L., Song C. W., Waddick K., Myers D. E., Kirihara J., Ledbetter J. A., Schieven G. L. Ionizing radiation stimulates unidentified tyrosine-specific protein kinases in human B-lymphocyte precursors, triggering apoptosis and clonogenic cell death. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9005–9009. doi: 10.1073/pnas.89.19.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand-Württenberger A., Schoel B., Ivanyi J., Kaufmann S. H. Surface expression by mononuclear phagocytes of an epitope shared with mycobacterial heat shock protein 60. Eur J Immunol. 1991 Apr;21(4):1089–1092. doi: 10.1002/eji.1830210437. [DOI] [PubMed] [Google Scholar]
- Ward J. F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Warters R. L. Radiation-induced apoptosis in a murine T-cell hybridoma. Cancer Res. 1992 Feb 15;52(4):883–890. [PubMed] [Google Scholar]
- Williams D. J., Newson J., Naessens J. Quantitation of bovine immunoglobulin isotypes and allotypes using monoclonal antibodies. Vet Immunol Immunopathol. 1990 Mar;24(3):267–283. doi: 10.1016/0165-2427(90)90042-q. [DOI] [PubMed] [Google Scholar]


