Abstract
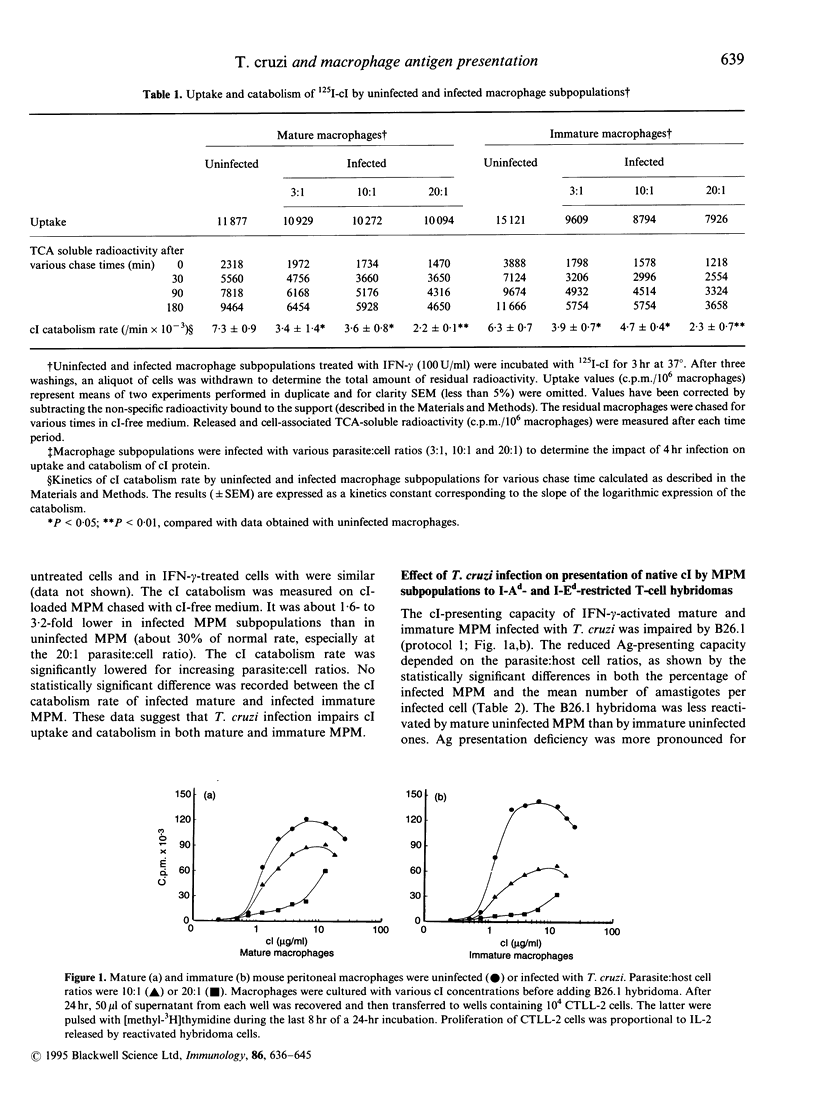
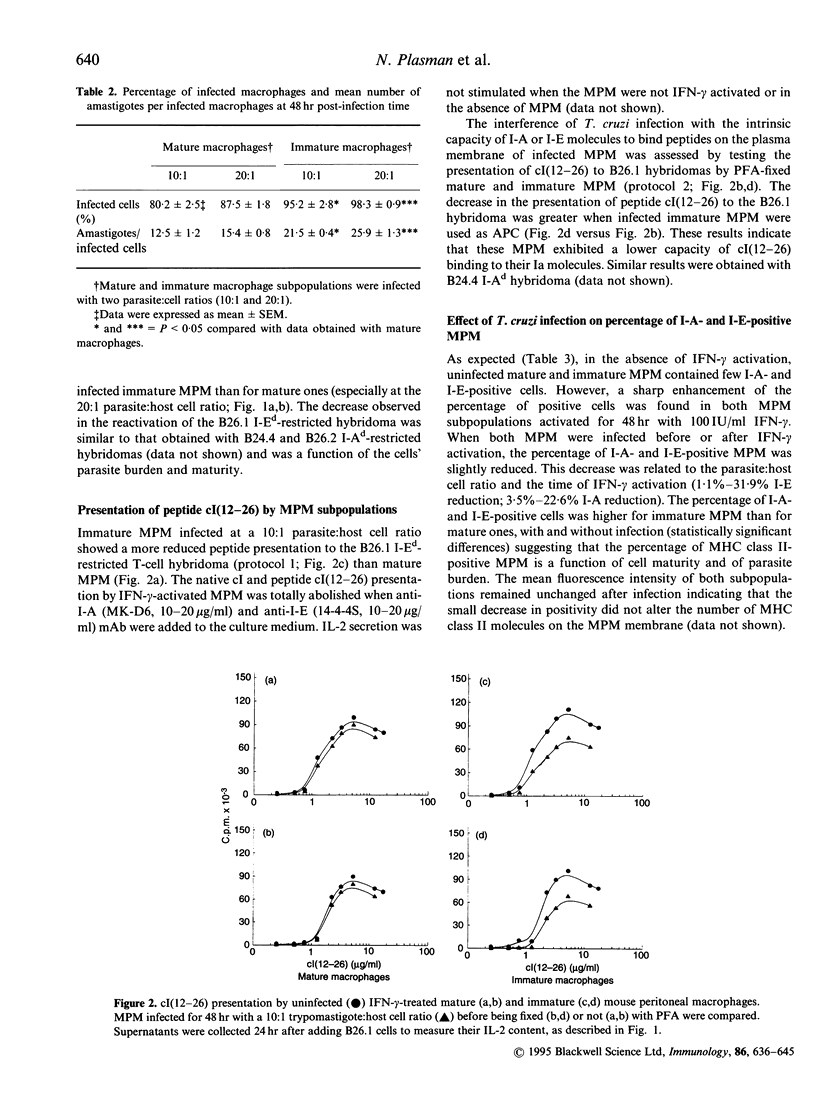
The effect of Trypanosoma cruzi infection on the ability of mature and immature murine peritoneal macrophage (MPM) subpopulations to catabolize the bacteriophage lambda repressor cI protein (cI) has been investigated. The capacity of infected MPM to present the cI and to stimulate various CD4+, I-Ad- or I-Ed-restricted T-cell hybridomas specific for cI was also assessed. Our results show that the radioiodinated cI uptake and catabolism decreased sharply after infection of MPM with T. cruzi. A cI presentation deficiency appeared in mature and immature MPM infected with T. cruzi trypomastigotes. The ability of infected MPM to bind immunogenic cI (12-26) peptides to the plasma membrane Ia molecules was also altered, especially in immature MPM, as shown with paraformaldehyde prefixed MPM, suggesting that these MPM only have a few functional Ia molecules on their membrane. The reduced capacity of cI presentation to the I-Ed-restricted B26.1 hybridomas by infected MPM subpopulations was comparable to that of the I-Ad-restricted B24.4 and B26.2 T cells. The percentage of major histocompatibility complex (MHC) class II-positive MPM was also reduced after T. cruzi infection. The percentage of positive interleukin-2 receptor (IL-2R) MPM was sharply lowered in infected cells, even with a pre- or a post-interferon-gamma (IFN-gamma) activation. Finally, inhibition of prostaglandin with indomethacin, or of nitric oxide with N-monomethyl-L-arginine, or of tumour necrosis factor-alpha (TNF-alpha) with specific monoclonal antibodies did not restore the cI presentation capacities of the MPM subpopulations. Taken together, these results suggest that T. cruzi infection induces a reduced capacity for macrophages to take up and catabolize antigen, resulting in a deficient antigen processing and presentation of the derived immunogenic peptides to specific CD4+ T-helper type-1 cell hybridomas. The decreased cI presenting capacity was a function of the cell's burden and maturity.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Nagy Z. A. Peptide competition for antigen presentation. Immunol Today. 1990 Jan;11(1):21–24. doi: 10.1016/0167-5699(90)90006-u. [DOI] [PubMed] [Google Scholar]
- Andrews N. W., Webster P. Phagolysosomal escape by intracellular pathogens. Parasitol Today. 1991 Dec;7(12):335–340. doi: 10.1016/0169-4758(91)90212-7. [DOI] [PubMed] [Google Scholar]
- Behbehani K., Pan S. C., Unanue E. R. Marked increase in Ia-bearing macrophages during Trypanosoma cruzi infection. Clin Immunol Immunopathol. 1981 May;19(2):190–195. doi: 10.1016/0090-1229(81)90062-3. [DOI] [PubMed] [Google Scholar]
- Bonaldo M. C., d'Escoffier L. N., Salles J. M., Goldenberg S. Characterization and expression of proteases during Trypanosoma cruzi metacyclogenesis. Exp Parasitol. 1991 Jul;73(1):44–51. doi: 10.1016/0014-4894(91)90006-i. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Bertoglio J. H. Prostaglandins E as modulators of the immune response. Lymphokine Res. 1988 Fall;7(3):237–245. [PubMed] [Google Scholar]
- Delovitch T. L., Semple J. W., Phillips M. L. Influence of antigen processing on immune responsiveness. Immunol Today. 1988 Jul-Aug;9(7-8):216–218. doi: 10.1016/0167-5699(88)91217-0. [DOI] [PubMed] [Google Scholar]
- Ferro T. J., Kern J. A., Elias J. A., Kamoun M., Daniele R. P., Rossman M. D. Alveolar macrophages, blood monocytes, and density-fractionated alveolar macrophages differ in their ability to promote lymphocyte proliferation to mitogen and antigen. Am Rev Respir Dis. 1987 Mar;135(3):682–687. doi: 10.1164/arrd.1987.135.3.682. [DOI] [PubMed] [Google Scholar]
- Fraser J. D., Newton M. E., Weiss A. CD28 and T cell antigen receptor signal transduction coordinately regulate interleukin 2 gene expression in response to superantigen stimulation. J Exp Med. 1992 Apr 1;175(4):1131–1134. doi: 10.1084/jem.175.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruth U., Solioz N., Louis J. A. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993 Mar 1;150(5):1857–1864. [PubMed] [Google Scholar]
- Green L. M., Reade J. L., Ware C. F. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods. 1984 May 25;70(2):257–268. doi: 10.1016/0022-1759(84)90190-x. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Avrameas S. Polyacrylamide-agarose beads for the preparation of effective immunoabsorbents. J Immunol Methods. 1976;11(2):129–133. doi: 10.1016/0022-1759(76)90140-x. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Geuze H. J. Immunogenic peptides bind to class II MHC molecules in an early lysosomal compartment. J Immunol. 1993 Oct 15;151(8):3988–3998. [PubMed] [Google Scholar]
- Lai M. Z., Ross D. T., Guillet J. G., Briner T. J., Gefter M. L., Smith J. A. T lymphocyte response to bacteriophage lambda repressor cI protein. Recognition of the same peptide presented by Ia molecules of different haplotypes. J Immunol. 1987 Dec 15;139(12):3973–3980. [PubMed] [Google Scholar]
- Ley V., Robbins E. S., Nussenzweig V., Andrews N. W. The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med. 1990 Feb 1;171(2):401–413. doi: 10.1084/jem.171.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R., Heirwegh K., Neirynck A., Remels L., Van Heuverswyn H., De Baetselier P. Generation and characterization of a neutralizing rat anti-rmTNF-alpha monoclonal antibody. Immunology. 1990 Oct;71(2):218–223. [PMC free article] [PubMed] [Google Scholar]
- Mbawuike I. N., Herscowitz H. B. Relationship between ineffective antigen presentation by murine alveolar macrophages and their immunosuppressive function. Immunology. 1988 May;64(1):61–67. [PMC free article] [PubMed] [Google Scholar]
- Metz G., Carlier Y., Vray B. Trypanosoma cruzi upregulates nitric oxide release by IFN-gamma-preactivated macrophages, limiting cell infection independently of the respiratory burst. Parasite Immunol. 1993 Dec;15(12):693–699. doi: 10.1111/j.1365-3024.1993.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Osawa H., Diamantstein T. A rat monoclonal antibody that binds specifically to mouse T lymphoblasts and inhibits IL 2 receptor functions: a putative anti-IL 2 receptor antibody. J Immunol. 1984 May;132(5):2445–2450. [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Plasman N., Metz G., Vray B. Interferon-gamma-activated immature macrophages exhibit a high Trypanosoma cruzi infection rate associated with a low production of both nitric oxide and tumor necrosis factor-alpha. Parasitol Res. 1994;80(7):554–558. doi: 10.1007/BF00933002. [DOI] [PubMed] [Google Scholar]
- Plasman N., Vray B. Immunophenotyping of murine peritoneal macrophages and lymphocytes by flow cytometry. J Immunol Methods. 1994 Sep 14;174(1-2):123–131. doi: 10.1016/0022-1759(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Plasman N., Vray B. Mouse peritoneal macrophages: characterization of functional subsets following Percoll density gradients. Res Immunol. 1993 Feb;144(2):151–163. doi: 10.1016/0923-2494(93)80070-f. [DOI] [PubMed] [Google Scholar]
- Prina E., Jouanne C., de Souza Lão S., Szabo A., Guillet J. G., Antoine J. C. Antigen presentation capacity of murine macrophages infected with Leishmania amazonensis amastigotes. J Immunol. 1993 Aug 15;151(4):2050–2061. [PubMed] [Google Scholar]
- Rottenberg M. E., Sunnemark D., Leandersson T., Orn A. Organ-specific regulation of interferon-gamma, interleukin-2 and interleukin-2 receptor during murine infection with Trypanosoma cruzi. Scand J Immunol. 1993 May;37(5):559–568. doi: 10.1111/j.1365-3083.1993.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Colon S., Kappler J. W., Marrack P., Grey H. M. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984 Oct;133(4):2067–2074. [PubMed] [Google Scholar]
- Tanowitz H. B., Kirchhoff L. V., Simon D., Morris S. A., Weiss L. M., Wittner M. Chagas' disease. Clin Microbiol Rev. 1992 Oct;5(4):400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trannoy E., Manser T., Cole M. D., Daley M. J. Monoclonal c-myc transformed macrophage cell lines. I. Heterogeneity in ability to process and present antigen. J Immunol. 1993 Sep 15;151(6):3042–3056. [PubMed] [Google Scholar]
- Tzehoval E., De Baetselier P., Feldman M., Segal S. The peritoneal antigen-presenting macrophage: control and immunogenic properties of distinct subpopulations. Eur J Immunol. 1981 Apr;11(4):323–328. doi: 10.1002/eji.1830110411. [DOI] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- Vray B., De Baetselier P., Ouaissi A., Carlier Y. Trypanosoma cruzi but not Trypanosoma brucei fails to induce a chemiluminescent signal in a macrophage hybridoma cell line. Infect Immun. 1991 Sep;59(9):3303–3308. doi: 10.1128/iai.59.9.3303-3308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E., Warner N. L., Chesnut R., Kappler J., Marrack P. Antigen-specific. I region-restricted interactions in vitro between tumor cell lines and T cell hybridomas. J Immunol. 1982 May;128(5):2164–2169. [PubMed] [Google Scholar]
- Walker W. S. Differential antigen presentation by cloned populations of mouse splenic macrophages. J Immunol. 1989 Oct 1;143(7):2142–2145. [PubMed] [Google Scholar]


