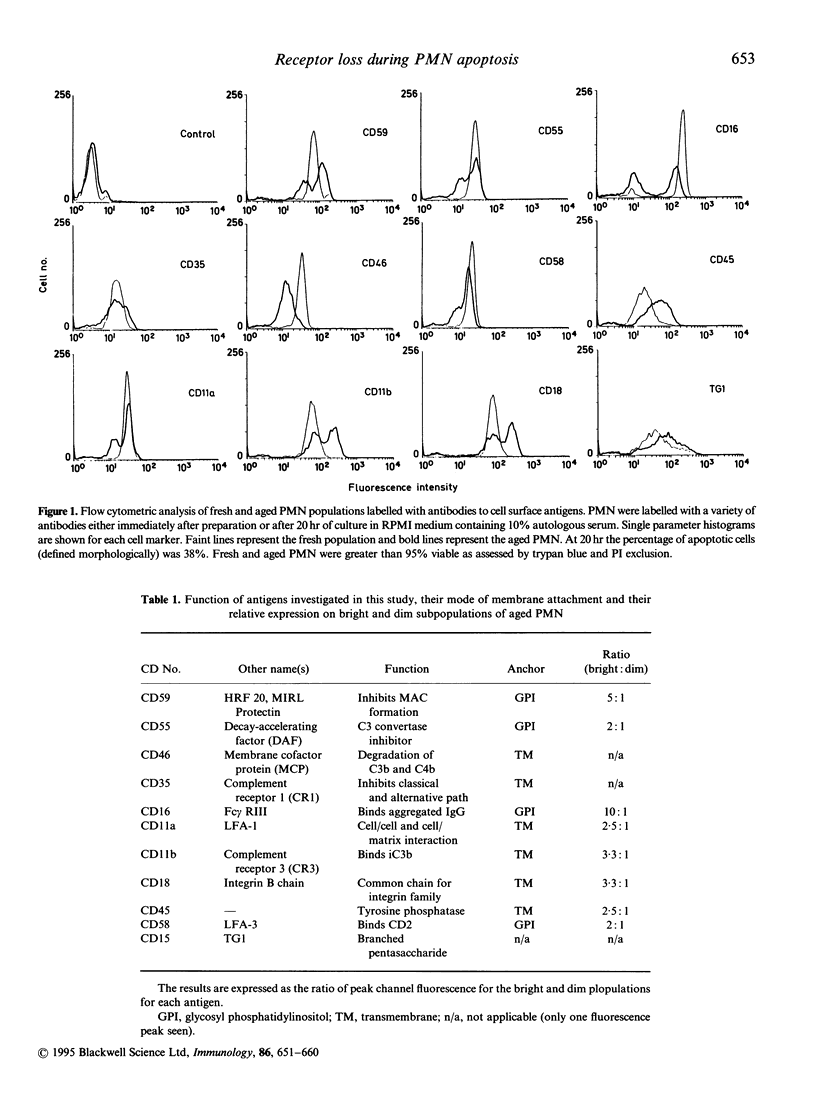
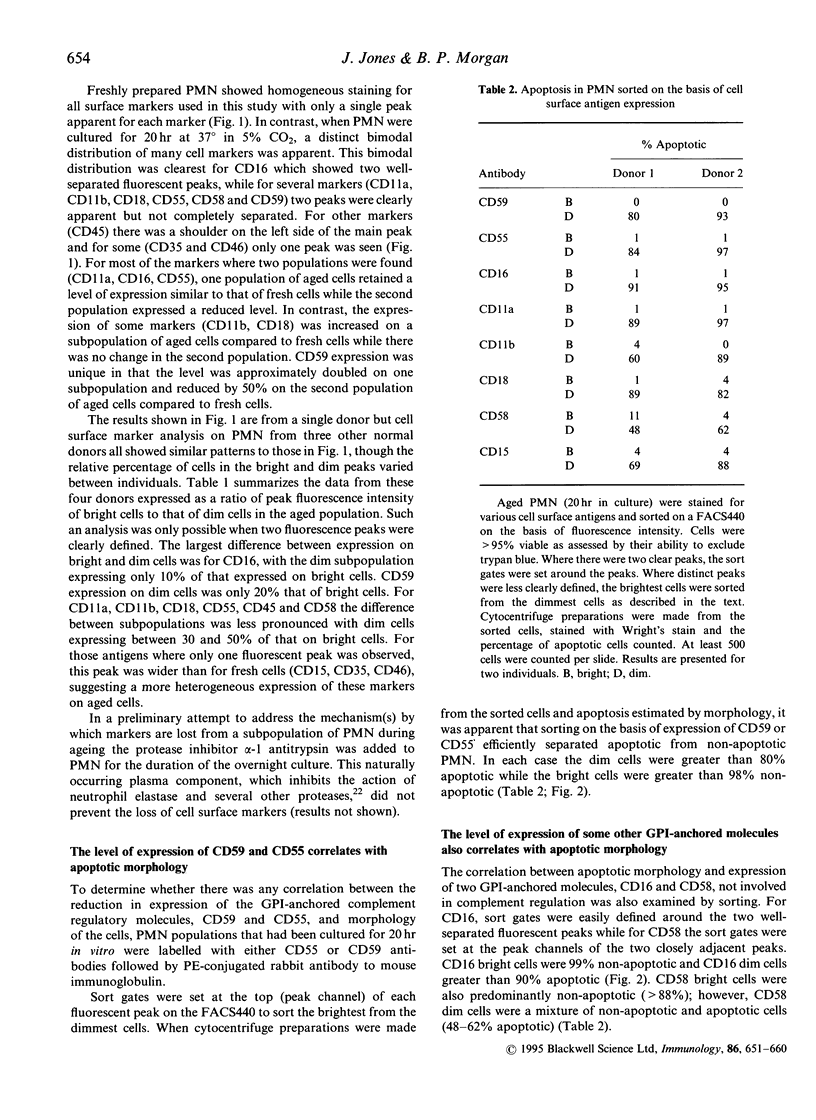
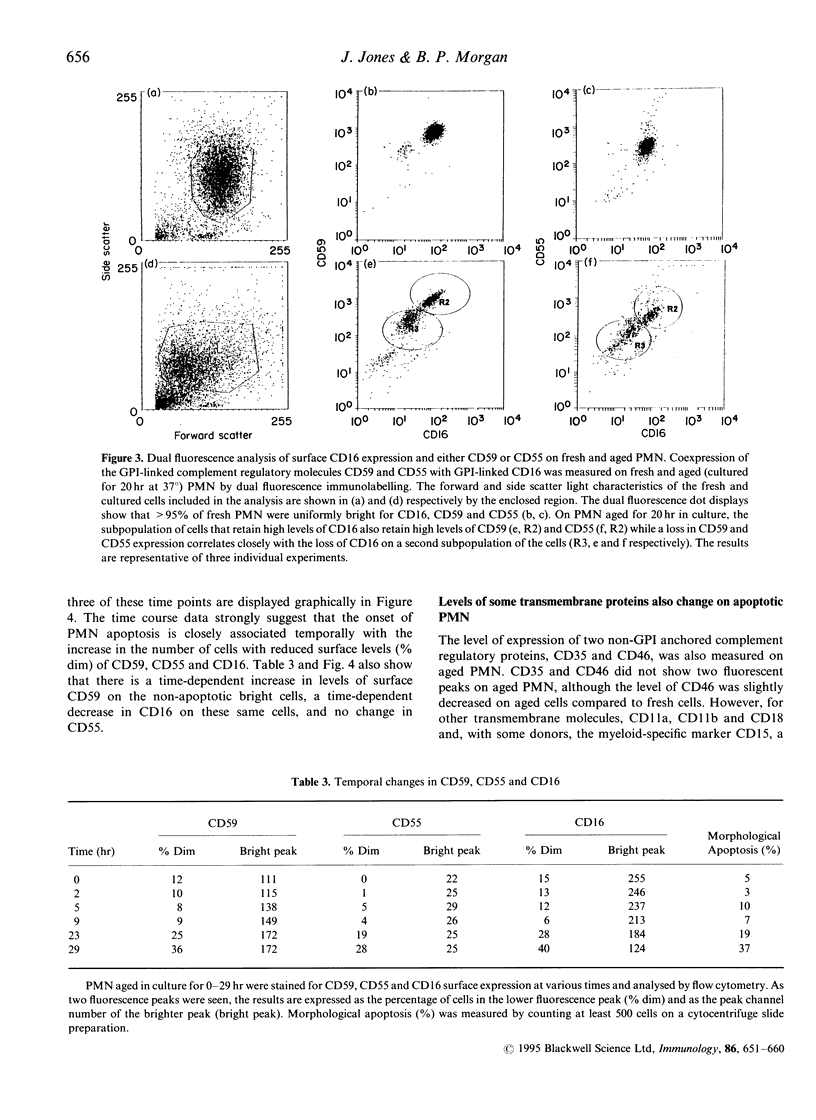
Abstract
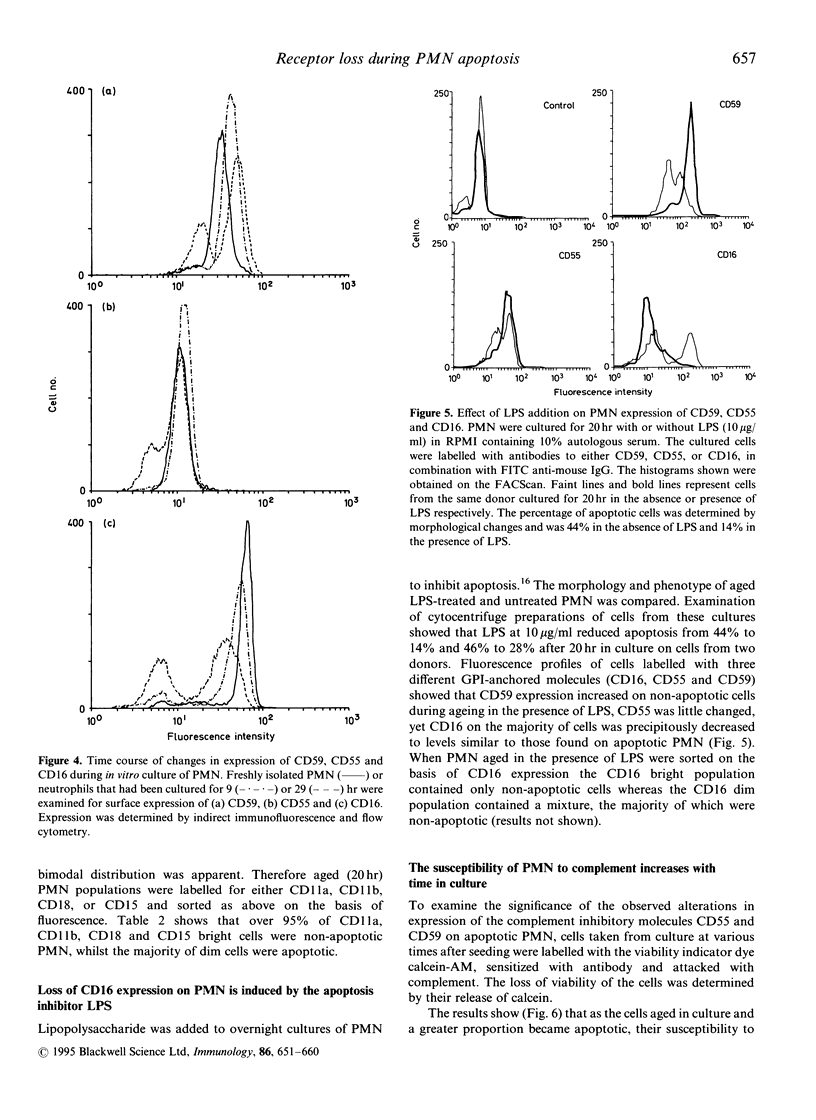
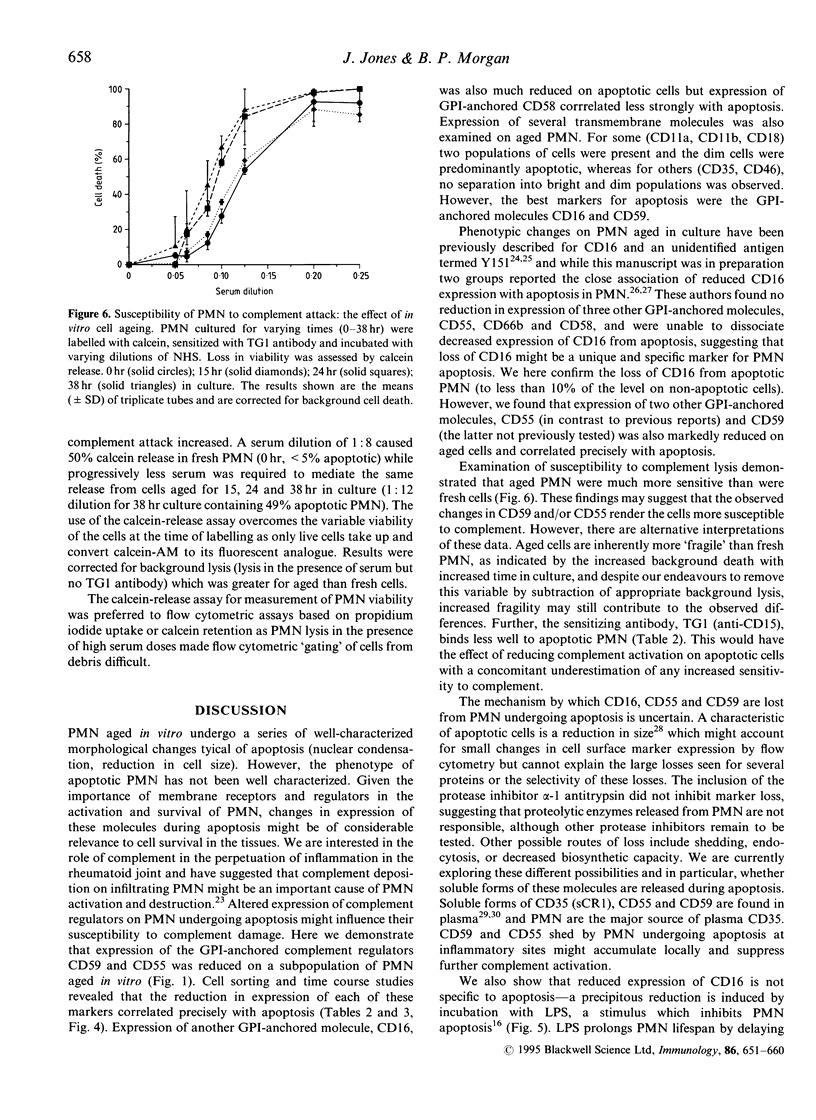
Human polymorphonuclear leucocytes (PMN) express proteins that protect them from damage by homologous complement. Protection may be particularly important when these cells migrate to inflammatory sites where complement activation is taking place. Resolution of inflammation involves removal of these PMN. The major mechanism of removal is likely to involve PMN apoptosis followed by recognition and engulfment by macrophages. However, little attention has been paid to the possible relevance of apoptosis to PMN susceptibility to immune effectors. Here we describe a reduction in cell surface expression of two complement regulatory proteins, CD59, an inhibitor of the membrane attack complex and CD55 (decay accelerating factor), an inhibitor of the C3/C5 convertase, on a subpopulation of PMN aged in culture. Loss of these proteins, both attached to the membrane by glycosyl phosphatidylinositol (GPI) anchors, correlated closely with the appearance of apoptotic morphology. We also observed a marked reduction in expression of the GPI-anchored molecule CD16 on apoptotic PMN. Reduced expression of membrane proteins was not confined to those anchored through GPI--several transmembrane molecules including CD11a CD11b and CD18 were also reduced on apoptotic PMN, whilst other were little changed (CD35, CD46). The precipitous fall in CD16 surface expression on PMN was not specific for apoptosis--in vitro incubation of PMN with lipopolysaccharide-inhibited apoptosis but caused a reduction in CD16 expression to 'apoptotic' levels.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aida Y., Pabst M. J. Neutrophil responses to lipopolysaccharide. Effect of adherence on triggering and priming of the respiratory burst. J Immunol. 1991 Feb 15;146(4):1271–1276. [PubMed] [Google Scholar]
- Allen P. D., Bustin S. A., Newland A. C. The role of apoptosis (programmed cell death) in haemopoiesis and the immune system. Blood Rev. 1993 Mar;7(1):63–73. doi: 10.1016/0268-960x(93)90025-y. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Linch D., Delia D. Isolation of human haematopoietic progenitor cells using monoclonal antibodies. Nature. 1980 Sep 25;287(5780):332–333. doi: 10.1038/287332a0. [DOI] [PubMed] [Google Scholar]
- Dahinden C., Fehr J. Granulocyte activation by endotoxin. II. Role of granulocyte adherence, aggregation, and effect of cytochalasin B, and comparison with formylated chemotactic peptide-induced stimulation. J Immunol. 1983 Feb;130(2):863–868. [PubMed] [Google Scholar]
- Dransfield I., Buckle A. M., Savill J. S., McDowall A., Haslett C., Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J Immunol. 1994 Aug 1;153(3):1254–1263. [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Fittschen C., Sandhaus R. A., Worthen G. S., Henson P. M. Bacterial lipopolysaccharide enhances chemoattractant-induced elastase secretion by human neutrophils. J Leukoc Biol. 1988 Jun;43(6):547–556. doi: 10.1002/jlb.43.6.547. [DOI] [PubMed] [Google Scholar]
- Forsyth K. D., Levinsky R. J. Preparative procedures of cooling and re-warming increase leukocyte integrin expression and function on neutrophils. J Immunol Methods. 1990 Apr 17;128(2):159–163. doi: 10.1016/0022-1759(90)90206-b. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin A., Taylor M., Bernhagen J., Shakoor Z., Mayall S., Noble G., McCarthy D. A method of preparing blood leucocytes for flow cytometry which prevents upregulation of leucocyte integrins. J Immunol Methods. 1992 Feb 5;146(2):219–228. doi: 10.1016/0022-1759(92)90231-h. [DOI] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Homburg C. H., de Haas M., von dem Borne A. E., Verhoeven A. J., Reutelingsperger C. P., Roos D. Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995 Jan 15;85(2):532–540. [PubMed] [Google Scholar]
- Ichinose Y., Hara N., Ohta M., Aso H., Chikama H., Kawasaki M., Kubota I., Shimizu T., Yagawa K. Recombinant granulocyte colony-stimulating factor and lipopolysaccharide maintain the phenotype of and superoxide anion generation by neutrophils. Infect Immun. 1990 Jun;58(6):1647–1652. doi: 10.1128/iai.58.6.1647-1652.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J., Laffafian I., Cooper A. M., Williams B. D., Morgan B. P. Expression of complement regulatory molecules and other surface markers on neutrophils from synovial fluid and blood of patients with rheumatoid arthritis. Br J Rheumatol. 1994 Aug;33(8):707–712. doi: 10.1093/rheumatology/33.8.707. [DOI] [PubMed] [Google Scholar]
- Kemp P. A., Spragg J. H., Brown J. C., Morgan B. P., Gunn C. A., Taylor P. W. Immunohistochemical determination of complement activation in joint tissues of patients with rheumatoid arthritis and osteoarthritis using neoantigen-specific monoclonal antibodies. J Clin Lab Immunol. 1992;37(4):147–162. [PubMed] [Google Scholar]
- Lee A., Whyte M. K., Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993 Oct;54(4):283–288. [PubMed] [Google Scholar]
- Meagher L. C., Savill J. S., Baker A., Fuller R. W., Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J Leukoc Biol. 1992 Sep;52(3):269–273. [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnes T. E. Quantification of the C3d split products of human complement by a sensitive enzyme-linked immunosorbent assay. Scand J Immunol. 1985 Jun;21(6):607–613. doi: 10.1111/j.1365-3083.1985.tb01851.x. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Campbell A. K. The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J. 1985 Oct 1;231(1):205–208. doi: 10.1042/bj2310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Daniels R. H., Williams B. D. Measurement of terminal complement complexes in rheumatoid arthritis. Clin Exp Immunol. 1988 Sep;73(3):473–478. [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15(4):369–396. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- Nurcombe H. L., Bucknall R. C., Edwards S. W. Activation of the neutrophil myeloperoxidase-H2O2 system by synovial fluid isolated from patients with rheumatoid arthritis. Ann Rheum Dis. 1991 Apr;50(4):237–242. doi: 10.1136/ard.50.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. Activation of the complement system in rheumatoid synovitis. Fed Proc. 1973 Feb;32(2):134–137. [PubMed] [Google Scholar]
- Rumfeld W. R., Morgan B. P., Campbell A. K. The ninth complement component in rheumatoid arthritis, Behçet's disease and other rheumatic diseases. Br J Rheumatol. 1986 Aug;25(3):266–270. doi: 10.1093/rheumatology/25.3.266. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Kopicky J. A., Wigley F. M., Shin M. L., Frank M. M., Joiner K. A. Membrane attack complex of complement in rheumatoid synovial tissue demonstrated by immunofluorescent microscopy. J Rheumatol. 1986 Dec;13(6):1028–1034. [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. Macrophage recognition of senescent neutrophils. Clin Sci (Lond) 1992 Dec;83(6):649–655. doi: 10.1042/cs0830649. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Yoshida S., Taniguchi H., Qin M. H., Miyamoto H., Mizuguchi Y. Lipopolysaccharide and granulocyte colony-stimulating factor delay neutrophil apoptosis and ingestion by guinea pig macrophages. Infect Immun. 1993 May;61(5):1972–1979. doi: 10.1128/iai.61.5.1972-1979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]