Abstract
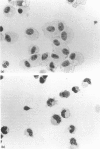
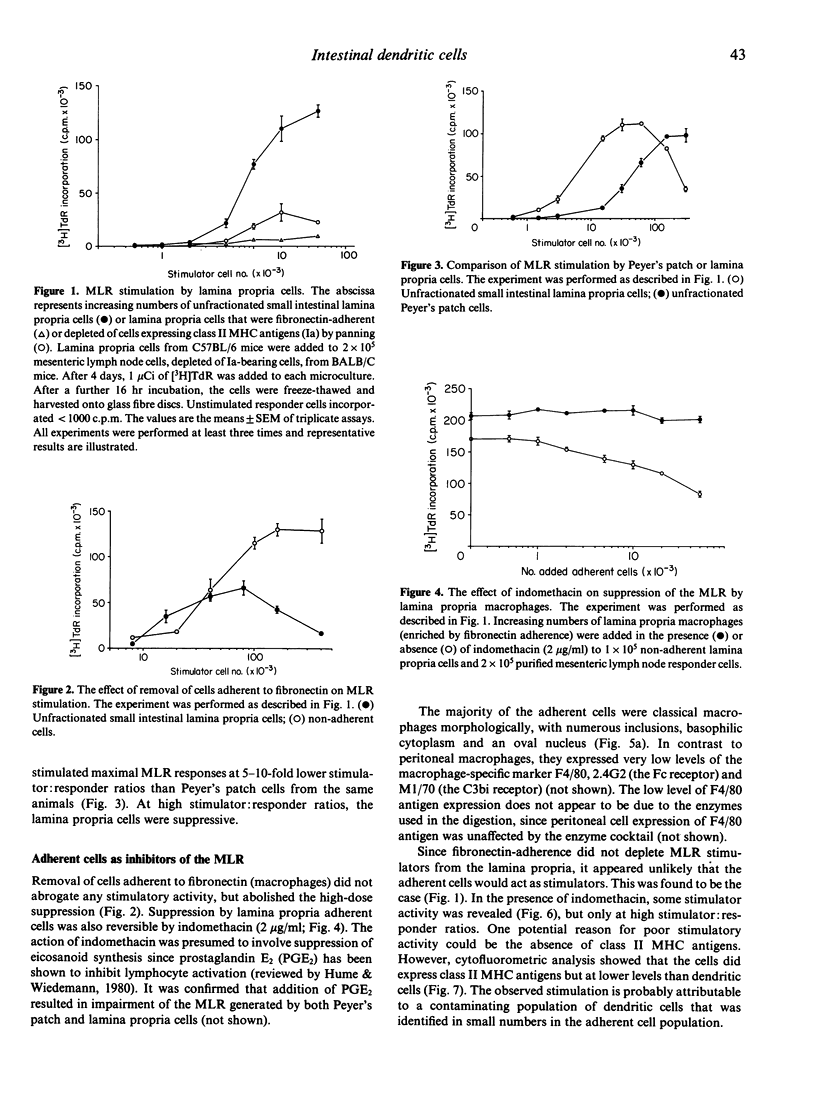
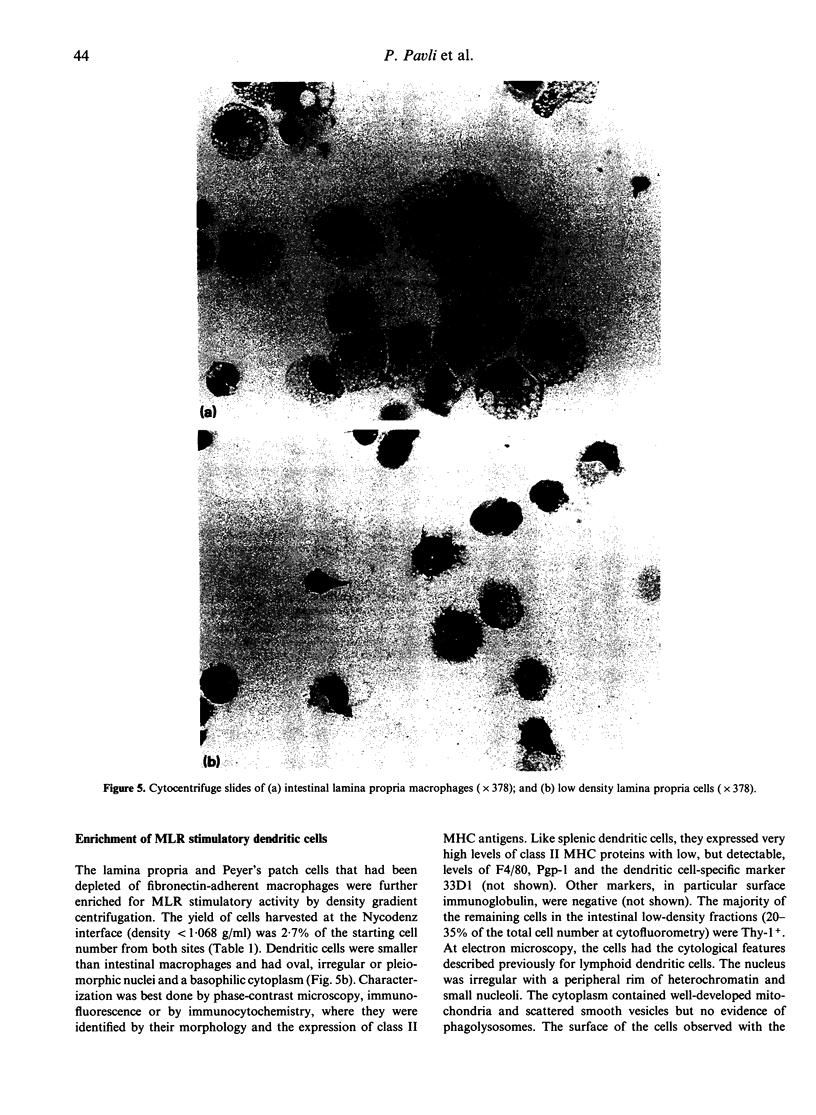
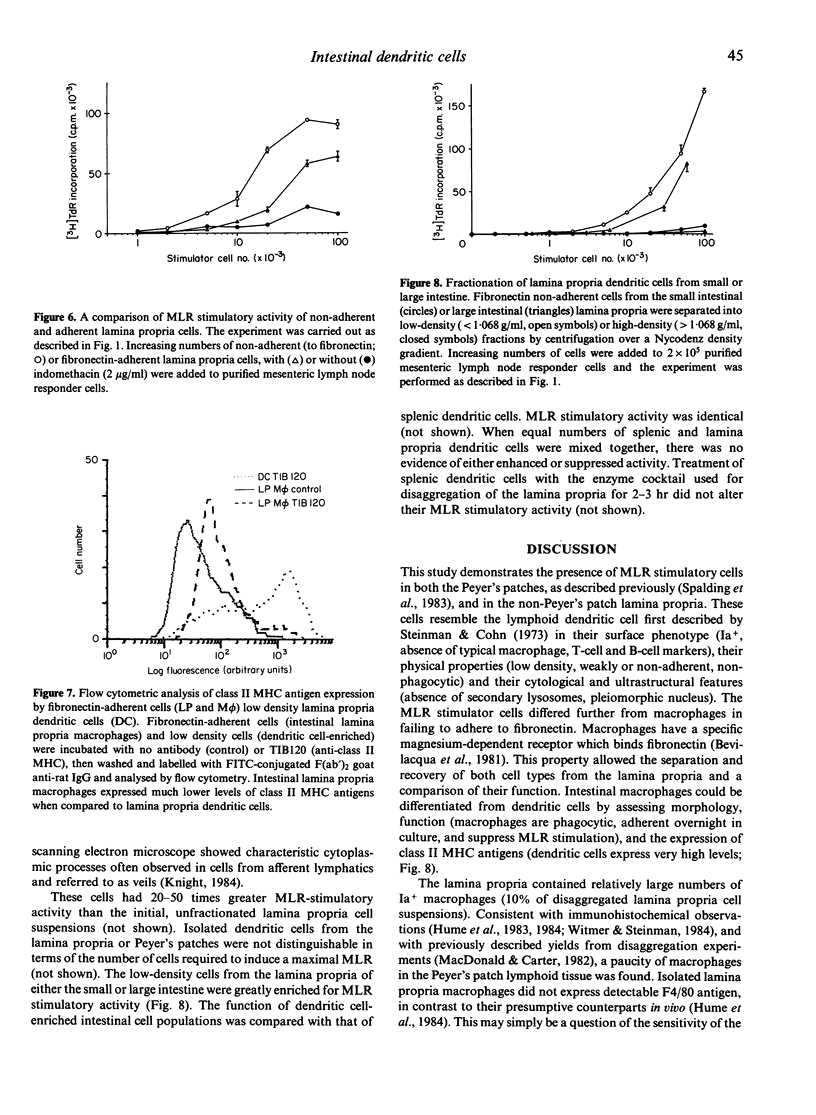
A method was developed for the isolation of antigen-presenting dendritic cells, and macrophages, from mouse intestinal lamina propria and Peyer's patches. Peyer's patches, and the lamina propria of both the small and large intestine, contained cells with potent stimulatory activity in the allogeneic mixed leucocyte reaction. These cells were separated from macrophages by fibronectin adherence and further enriched by density centrifugation. The isolated stimulatory cells expressed high levels of class II major histocompatibility complex (MHC) antigens, and resembled splenic dendritic cells in both morphology and function. Macrophages were recovered from the lamina propria but not Peyer's patches. These cells also expressed class II MHC antigens, but failed to stimulate the mixed leucocyte reaction and, instead, induced indomethacin-sensitive suppression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Britz J. S., Askenase P. W., Ptak W., Steinman R. M., Gershon R. K. Specialized antigen-presenting cells. Splenic dendritic cells and peritoneal-exudate cells induced by mycobacteria activate effector T cells that are resistant to suppression. J Exp Med. 1982 May 1;155(5):1344–1356. doi: 10.1084/jem.155.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucana C. D., Hoyer L. C., Schroit A. J., Kleinerman E., Fidler I. J. Ultrastructural studies of the interaction between liposome-activated human blood monocytes and allogeneic tumor cells in vitro. Am J Pathol. 1983 Jul;112(1):101–111. [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Antigen presenting cells and mechanisms of antigen presentation. Crit Rev Immunol. 1985;5(3):263–316. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Enders G., Gottwald T., Brendel W. Induction of oral tolerance in rats without Peyer's patches. Immunology. 1986 Jun;58(2):311–314. [PMC free article] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Heufler C., Koch F., Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988 Feb 1;167(2):700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., O'Leary C., Krska K., Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985 Dec;62(3):586–593. [PMC free article] [PubMed] [Google Scholar]
- Hughes E. N., Colombatti A., August J. T. Murine cell surface glycoproteins. Purification of the polymorphic Pgp-1 antigen and analysis of its expression on macrophages and other myeloid cells. J Biol Chem. 1983 Jan 25;258(2):1014–1021. [PubMed] [Google Scholar]
- Hume D. A., Allan W., Hogan P. G., Doe W. F. Immunohistochemical characterisation of macrophages in human liver and gastrointestinal tract: expression of CD4, HLA-DR, OKM1, and the mature macrophage marker 25F9 in normal and diseased tissue. J Leukoc Biol. 1987 Nov;42(5):474–484. doi: 10.1002/jlb.42.5.474. [DOI] [PubMed] [Google Scholar]
- Hume D. A. Immunohistochemical analysis of murine mononuclear phagocytes that express class II major histocompatibility antigens. Immunobiology. 1985 Dec;170(5):381–389. doi: 10.1016/S0171-2985(85)80062-0. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Perry V. H., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat Rec. 1984 Nov;210(3):503–512. doi: 10.1002/ar.1092100311. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Schuler G., Witmer M. D., Valinksy J., Atassi B., Steinman R. M. Immunologic properties of purified epidermal Langerhans cells. Distinct requirements for stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986 Aug 1;164(2):605–613. doi: 10.1084/jem.164.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Bofill M., Poulter L. W., Rawlings E., Burford G. D., Navarrete C., Ziegler A., Kelemen E. Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. J Immunol. 1986 Jun 15;136(12):4354–4361. [PubMed] [Google Scholar]
- Knight S. C. Veiled cells--"dendritic cells" of the peripheral lymph. Immunobiology. 1984 Dec;168(3-5):349–361. doi: 10.1016/S0171-2985(84)80122-9. [DOI] [PubMed] [Google Scholar]
- Koide S. L., Inaba K., Steinman R. M. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987 Feb 1;165(2):515–530. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S., Steinman R. M. Induction of murine interleukin 1: stimuli and responsive primary cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3802–3806. doi: 10.1073/pnas.84.11.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler R. I., Batchelor J. R. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982 Jan 1;155(1):31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Carter P. B. Isolation and functional characteristics of adherent phagocytic cells from mouse Peyer's patches. Immunology. 1982 Apr;45(4):769–774. [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Holt P. G., Papadimitriou J. M. Functional characteristics of the veiled cells in afferent lymph from the rat intestine. Immunology. 1986 Jul;58(3):379–387. [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- Nunez G., Ball E. J., Stastny P. Accessory cell function of human endothelial cells. I. A subpopulation of Ia positive cells is required for antigen presentation. J Immunol. 1983 Aug;131(2):666–673. [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Witmer M. D., Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R. A., King N. J., Blanden R. V. Comparison of functional properties of thymic and splenic dendritic cells. Cell Immunol. 1986 Oct 1;102(1):152–167. doi: 10.1016/0008-8749(86)90334-5. [DOI] [PubMed] [Google Scholar]
- Pugh C. W., MacPherson G. G., Steer H. W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983 Jun 1;157(6):1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding D. M., Koopman W. J., Eldridge J. H., McGhee J. R., Steinman R. M. Accessory cells in murine Peyer's patch. I. Identification and enrichment of a functional dendritic cell. J Exp Med. 1983 May 1;157(5):1646–1659. doi: 10.1084/jem.157.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J., MacDonald T. T., Isaacson P. G. Heterogeneity of non-lymphoid cells expressing HLA-D region antigens in human fetal gut. Clin Exp Immunol. 1987 Feb;67(2):415–424. [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer-Pack M. D., Olivier W., Valinsky J., Schuler G., Steinman R. M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987 Nov 1;166(5):1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer M. D., Steinman R. M. The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer's patch. Am J Anat. 1984 Jul;170(3):465–481. doi: 10.1002/aja.1001700318. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]