Abstract
It has been reported previously that environmental antigens such as intestinal microflora play an important role in an age-associated increase in number of T-cell receptor gamma/delta-bearing T cells. To extend the scope of this finding, this study examined the influences of local inflammation on the accumulation and/or proliferation of gamma/delta T cells in athymic nude mice injected subcutaneously with complete Freund's adjuvants (CFA). The inguinal lymph nodes (ILN) in nude mice injected with CFA in their hind footpads 7 days previously contained an increased number of CD3+CD4-CD8- cells. Increased levels of proliferative responses against syngeneic stimulator cells were noted in the LN cells of CFA-injected nude mice in association with increases in expression of gamma- and delta-chain gene messages. Furthermore, the LN cells showed an early proliferative response to purified protein derivative (PPD) from Mycobacterium bovis. These results suggest that local inflammation by CFA may induce functional gamma/delta T cells in nude mice, which may proliferate rapidly at the inflamed sites and represent a first line of defence in such animals against the invasion of various pathogens.
Full text
PDF

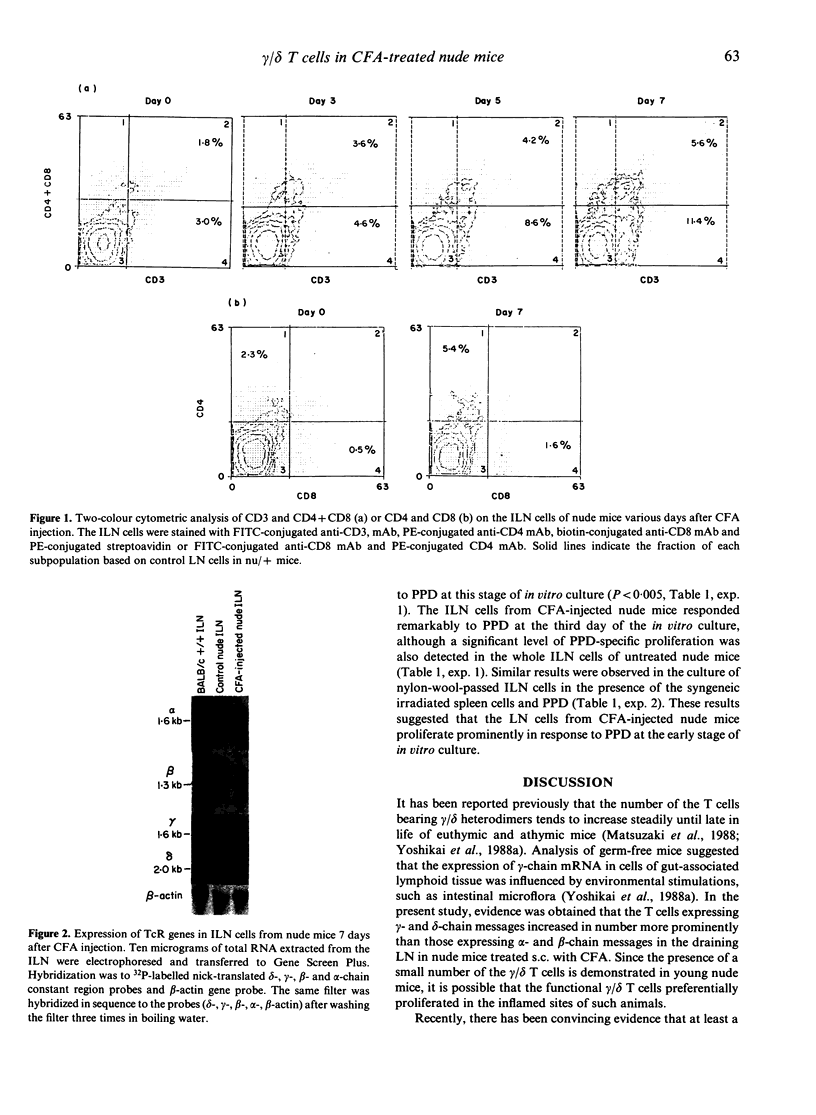
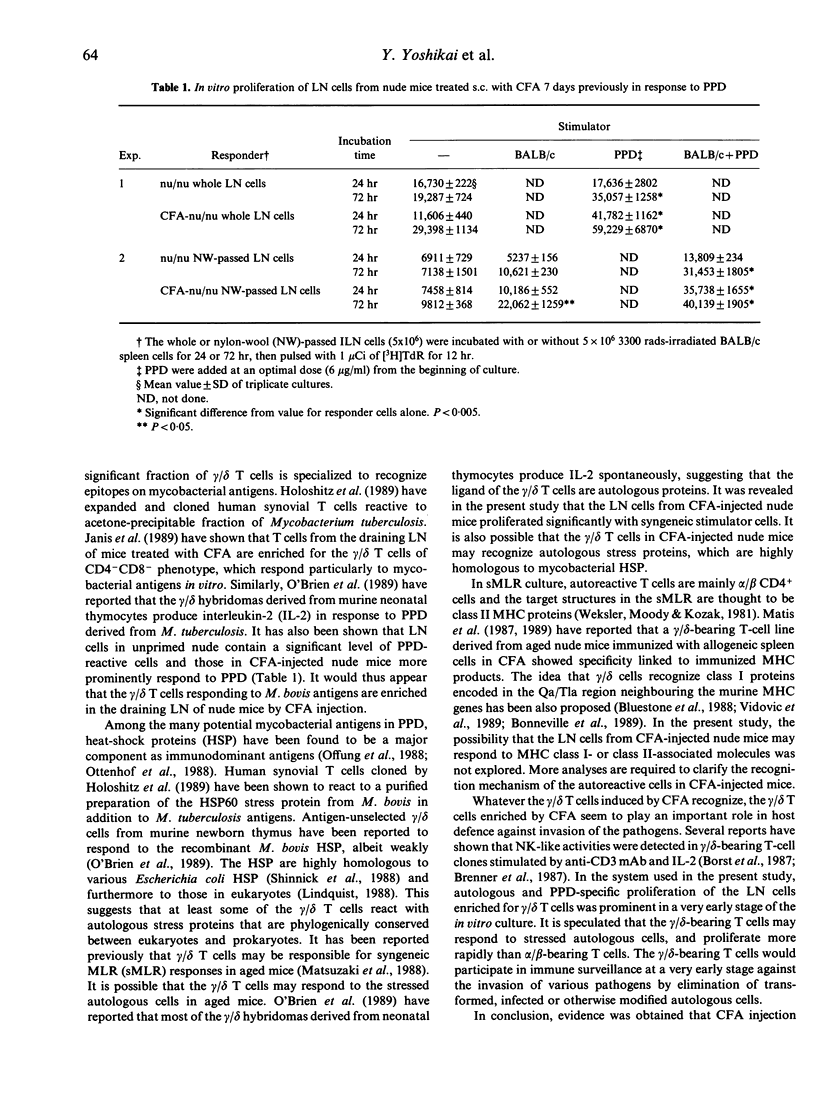

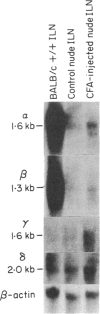
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bluestone J. A., Cron R. Q., Cotterman M., Houlden B. A., Matis L. A. Structure and specificity of T cell receptor gamma/delta on major histocompatibility complex antigen-specific CD3+, CD4-, CD8- T lymphocytes. J Exp Med. 1988 Nov 1;168(5):1899–1916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., Ito K., Krecko E. G., Itohara S., Kappes D., Ishida I., Kanagawa O., Janeway C. A., Murphy D. B., Tonegawa S. Recognition of a self major histocompatibility complex TL region product by gamma delta T-cell receptors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5928–5932. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J., van de Griend R. J., van Oostveen J. W., Ang S. L., Melief C. J., Seidman J. G., Bolhuis R. L. A T-cell receptor gamma/CD3 complex found on cloned functional lymphocytes. Nature. 1987 Feb 19;325(6106):683–688. doi: 10.1038/325683a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., Strominger J. L., Krangel M. S. The gamma delta T cell receptor. Adv Immunol. 1988;43:133–192. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Fowlkes B. J., Pardoll D. M. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Samaridis J., Pelkonen J. Progression of rearrangements at T cell receptor beta and gamma gene loci during athymic differentiation of bone marrow cells in vitro. Cell. 1988 Mar 25;52(6):821–829. doi: 10.1016/0092-8674(88)90424-2. [DOI] [PubMed] [Google Scholar]
- Iwamoto A., Rupp F., Ohashi P. S., Walker C. L., Pircher H., Joho R., Hengartner H., Mak T. W. T cell-specific gamma genes in C57BL/10 mice. Sequence and expression of new constant and variable region genes. J Exp Med. 1986 May 1;163(5):1203–1212. doi: 10.1084/jem.163.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kishihara K., Yoshikai Y., Matsuzaki G., Tomooka S., Nomoto K. "Radioresistant" intrathymic T cell precursors express T cell receptor C gamma 4- and C delta-specific gene messages. Eur J Immunol. 1988 Jun;18(6):841–847. doi: 10.1002/eji.1830180603. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Cron R., Bluestone J. A. Major histocompatibility complex-linked specificity of gamma delta receptor-bearing T lymphocytes. Nature. 1987 Nov 19;330(6145):262–264. doi: 10.1038/330262a0. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Fry A. M., Cron R. Q., Cotterman M. M., Dick R. F., Bluestone J. A. Structure and specificity of a class II MHC alloreactive gamma delta T cell receptor heterodimer. Science. 1989 Aug 18;245(4919):746–749. doi: 10.1126/science.2528206. [DOI] [PubMed] [Google Scholar]
- Matsuzaki G., Yoshikai Y., Kishihara K., Nomoto K., Yokokura T., Nomoto K. Age-associated increase in the expression of T cell antigen receptor gamma chain genes in mice. Eur J Immunol. 1988 Nov;18(11):1779–1784. doi: 10.1002/eji.1830181119. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Shinnick T. M., Houghten R. A., Kvalheim G., Degre M., Lundin K. E., Godal T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J Immunol. 1988 Oct 15;141(8):2749–2754. [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Kappler J. W., Marrack P. Tolerance to self antigens shapes the T-cell repertoire. Immunol Rev. 1989 Feb;107:125–139. doi: 10.1111/j.1600-065x.1989.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Weiss A., Miller J., Norcross M. A., Germain R. N. Specific antigen-Ia activation of transfected human T cells expressing murine Ti alpha beta-human T3 receptor complexes. Nature. 1987 Jan 8;325(7000):125–130. doi: 10.1038/325125a0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyonaga B., Mak T. W. Genes of the T-cell antigen receptor in normal and malignant T cells. Annu Rev Immunol. 1987;5:585–620. doi: 10.1146/annurev.iy.05.040187.003101. [DOI] [PubMed] [Google Scholar]
- Vidović D., Roglić M., McKune K., Guerder S., MacKay C., Dembić Z. Qa-1 restricted recognition of foreign antigen by a gamma delta T-cell hybridoma. Nature. 1989 Aug 24;340(6235):646–650. doi: 10.1038/340646a0. [DOI] [PubMed] [Google Scholar]
- Weksler M. E., Moody C. E., Jr, Kozak R. W. The autologous mixed-lymphocyte reaction. Adv Immunol. 1981;31:271–312. doi: 10.1016/s0065-2776(08)60923-2. [DOI] [PubMed] [Google Scholar]
- Wilson R. K., Lai E., Concannon P., Barth R. K., Hood L. E. Structure, organization and polymorphism of murine and human T-cell receptor alpha and beta chain gene families. Immunol Rev. 1988 Jan;101:149–172. doi: 10.1111/j.1600-065x.1988.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Matsuzaki G., Kishihara K., Nomoto K., Yokokura T., Nomoto K. Age-associated increase in the expression of T-cell antigen receptor gamma-chain gene in conventional and germfree mice. Infect Immun. 1988 Aug;56(8):2069–2074. doi: 10.1128/iai.56.8.2069-2074.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Matsuzaki G., Takeda Y., Ohga S., Kishihara K., Yuuki H., Nomoto K. Functional T cell receptor delta chain gene messages in athymic nude mice. Eur J Immunol. 1988 Jul;18(7):1039–1043. doi: 10.1002/eji.1830180711. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Reis M. D., Mak T. W. Athymic mice express a high level of functional gamma-chain but greatly reduced levels of alpha- and beta-chain T-cell receptor messages. Nature. 1986 Dec 4;324(6096):482–485. doi: 10.1038/324482a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M. Thymus and lymphohemopoietic cells: their role in T cell maturation in selection of T cells' H-2-restriction-specificity and in H-2 linked Ir gene control. Immunol Rev. 1978;42:224–270. doi: 10.1111/j.1600-065x.1978.tb00264.x. [DOI] [PubMed] [Google Scholar]