Abstract
This study evaluated the gene expression of tumour necrosis factor (TNF) and the molecular weight of the cytotoxic factor in a subline of a rat basophilic leukaemia cell line, RBL-2H3. After IgE receptor triggering with a specific antigen that was associated with histamine release, cytotoxic activity in the cell lysates and supernatants increased for 2 hr during the culture of RBL-2H3 cells. Furthermore, calcium ionophore A23187 could induce release of histamine and cytotoxic activity from RBL-2H3 cells. However, compound 48/80, lipopolysaccharide (LPS) and phorbol 12-myristate 13-acetate (PMA) were unable to induce the release of either histamine or cytotoxic activity from the cells. These data suggested that, at least in part, there was a common pathway in histamine release and production of cytotoxic activity. A protein synthesis inhibitor, cycloheximide, did not affect histamine release, but inhibited the induction of cytotoxic activity. This cytotoxic activity from RBL-2H3 cells was completely neutralized by anti-mouse TNF rabbit serum. With Northern blot analysis, mouse TNF cDNA probe could hybridize with RNA isolated from RBL-2H3 cells. TNF mRNA was induced as early as 1 hr after stimulation with specific antigen and decreased by 4 hr. Moreover, the molecular weight (MW) of the released cytotoxic factor from RBL-2H3 cells triggered with IgE receptors was approximately 17,000 by SDS-PAGE, which was compatible to that of TNF. Thus, it is concluded that the gene expression and production of TNF occurred in RBL-2H3 cells after IgE receptor triggering in association with histamine release, suggesting that TNF produced by basophils and mast cells may play an important role in allergic reaction through its wide range of biological activity.
Full text
PDF

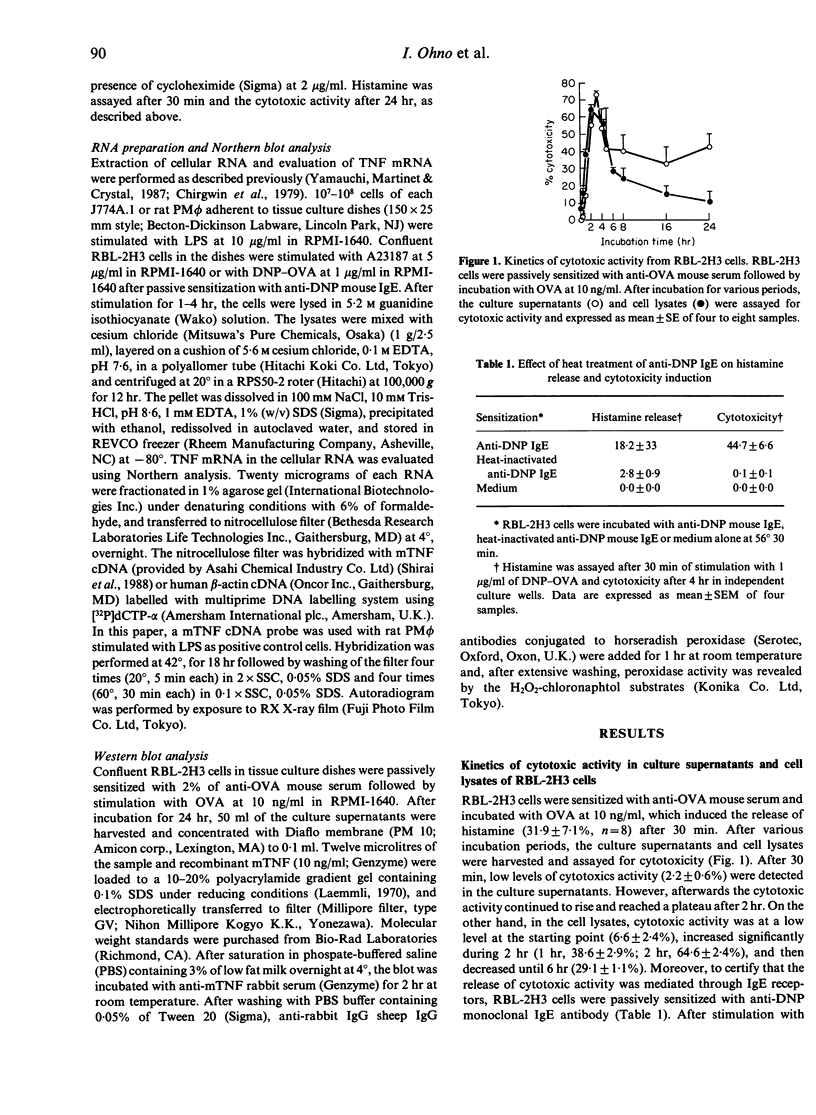
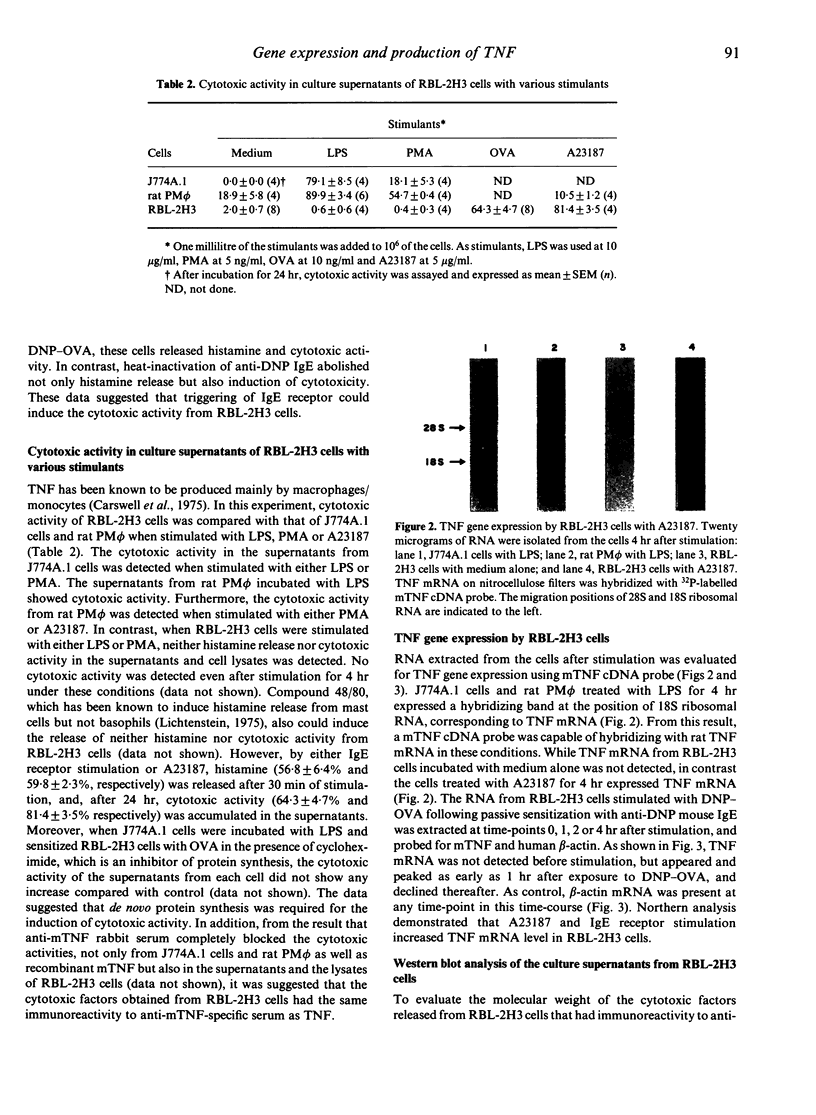
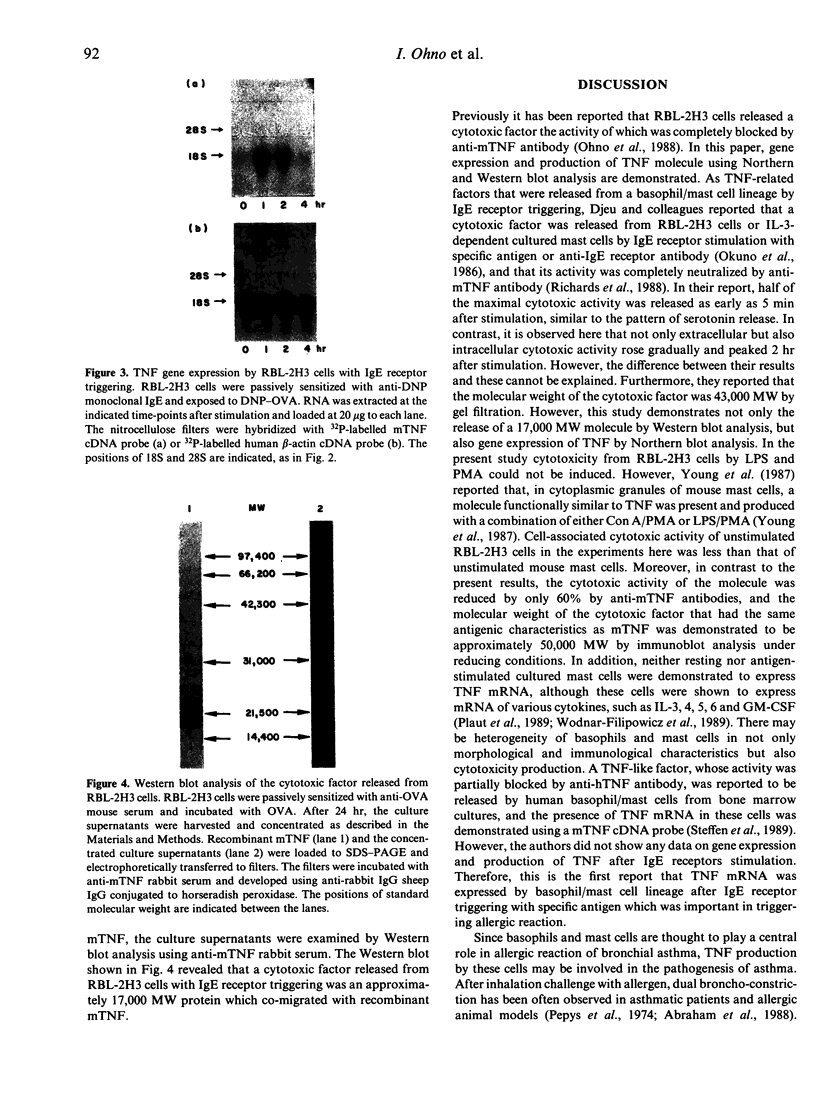

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. M., Stevenson J. S., Eldridge M., Garrido R., Nieves L. Nedocromil sodium in allergen-induced bronchial responses and airway hyperresponsiveness in allergic sheep. J Appl Physiol (1985) 1988 Sep;65(3):1062–1068. doi: 10.1152/jappl.1988.65.3.1062. [DOI] [PubMed] [Google Scholar]
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Leonhardt P. H. Aquanititave semimicro, semiautomated colorimetric assay for interferon. J Lab Clin Med. 1977 May;89(5):1036–1042. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale T. B., Wood D., Richerson H. B., Zehr B., Zavala D., Hunninghake G. W. Direct evidence of a role for mast cells in the pathogenesis of antigen-induced bronchoconstriction. J Clin Invest. 1987 Nov;80(5):1507–1511. doi: 10.1172/JCI113234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- De Monchy J. G., Kauffman H. F., Venge P., Koëter G. H., Jansen H. M., Sluiter H. J., De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985 Mar;131(3):373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- Haranaka K., Satomi N., Sakurai A., Nariuchi H. Purification and partial amino acid sequence of rabbit tumor necrosis factor. Int J Cancer. 1985 Sep 15;36(3):395–400. [PubMed] [Google Scholar]
- Ida S., Siraganian R. P., Notkins A. L. Cell-bound and circulating IgE antibody to herpes simplex virus. J Gen Virol. 1983 Mar;64(Pt 3):533–537. doi: 10.1099/0022-1317-64-3-533. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M. The mechanism of basophil histamine release induced by antigen and by the calcium ionophore A23187. J Immunol. 1975 Jun;114(6):1692–1699. [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Murphy K. R., Wilson M. C., Irvin C. G., Glezen L. S., Marsh W. R., Haslett C., Henson P. M., Larsen G. L. The requirement for polymorphonuclear leukocytes in the late asthmatic response and heightened airways reactivity in an animal model. Am Rev Respir Dis. 1986 Jul;134(1):62–68. doi: 10.1164/arrd.1986.134.1.62. [DOI] [PubMed] [Google Scholar]
- Ohno I., Tanno Y., Yamauchi K., Ida S., Takishima T. The production of tumor necrosis factor-alpha by rat basophilic leukemia cells with triggering IgE receptor. Tohoku J Exp Med. 1988 Oct;156(2):209–210. doi: 10.1620/tjem.156.209. [DOI] [PubMed] [Google Scholar]
- Ohno I., Tanno Y., Yamauchi K., Takishima T. Production of tumour necrosis factor by mastocytoma P815 cells. Immunology. 1990 Feb;69(2):312–315. [PMC free article] [PubMed] [Google Scholar]
- Okuno T., Takagaki Y., Pluznik D. H., Djeu J. Y. Natural cytotoxic (NC) cell activity in basophilic cells: release of NC-specific cytotoxic factor by IgE receptor triggering. J Immunol. 1986 Jun 15;136(12):4652–4658. [PubMed] [Google Scholar]
- Richards A. L., Okuno T., Takagaki Y., Djeu J. Y. Natural cytotoxic cell-specific cytotoxic factor produced by IL-3-dependent basophilic/mast cells. Relationship to TNF. J Immunol. 1988 Nov 1;141(9):3061–3066. [PubMed] [Google Scholar]
- Shirai T., Shimizu N., Shiojiri S., Horiguchi S., Ito H. Cloning and expression in Escherichia coli of the gene for mouse tumor necrosis factor. DNA. 1988 Apr;7(3):193–201. doi: 10.1089/dna.1988.7.193. [DOI] [PubMed] [Google Scholar]
- Silberstein D. S., David J. R. Tumor necrosis factor enhances eosinophil toxicity to Schistosoma mansoni larvae. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1055–1059. doi: 10.1073/pnas.83.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen M., Abboud M., Potter G. K., Yung Y. P., Moore M. A. Presence of tumour necrosis factor or a related factor in human basophil/mast cells. Immunology. 1989 Mar;66(3):445–450. [PMC free article] [PubMed] [Google Scholar]
- Warren M. K., Ralph P. Macrophage growth factor CSF-1 stimulates human monocyte production of interferon, tumor necrosis factor, and colony stimulating activity. J Immunol. 1986 Oct 1;137(7):2281–2285. [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Martinet Y., Crystal R. G. Modulation of fibronectin gene expression in human mononuclear phagocytes. J Clin Invest. 1987 Dec;80(6):1720–1727. doi: 10.1172/JCI113263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Liu C. C., Butler G., Cohn Z. A., Galli S. J. Identification, purification, and characterization of a mast cell-associated cytolytic factor related to tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9175–9179. doi: 10.1073/pnas.84.24.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]