Abstract
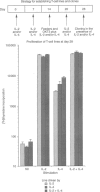
It is now well documented that the mode of primary stimulation in the mouse determines the ratio of Th1-, Th0- or Th2-type T cells obtained. In this paper we determine whether driving and cloning human peripheral blood mononuclear cells (PBMC) non-specifically with interleukin-2 (IL-2) and/or IL-4 and mitogen, will differentially select T cells for IL-10 production. The presence of IL-2 during culture (with or without IL-4) yielded predominantly Th1-type clones (81% of clones with IL-2 alone and 77% with IL-2 + IL-4) and a low frequency of Th0-type clones (19% of clones with IL-2 and 10% of clones with IL-2 + IL-4), whereas IL-4 alone increased the yield of the IL-4 producing Th0 (35%) clones. The proportions of IL-2-producing clones did not alter with treatment; however, the combination of IL-2 + IL-4 altered the proportions of IL-10-producing clones. 47% of IL-2-cultured cells and 51% of IL-4-cultured cells produced IL-10 respectively, whereas only 22% of clones driven with IL-2 + IL-4 produced detectable levels of IL-10. Thus in the preliminary experiments described here we have demonstrated that the cytokines used to initially drive out human T cells and maintain the T-cell growth can skew the Th1/Th2/Th0 cytokine profiles of the clones obtained from mitogen-stimulated cultures. Moreover and unexpectedly the proportion of IL-10-producing cells is altered. These results are important in interpreting analysis of the cytokine profiles of human T cells and raise questions concerning how we should derive T-cell clones for a cytokine analysis that truly reflects the in vivo situation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. B., Katsikis P. D., Chu C. Q., Thomssen H., Webb L. M., Maini R. N., Londei M., Feldmann M. High level of interleukin-10 production by the activated T cell population within the rheumatoid synovial membrane. Arthritis Rheum. 1995 Jul;38(7):946–952. doi: 10.1002/art.1780380710. [DOI] [PubMed] [Google Scholar]
- Correale J., Gilmore W., McMillan M., Li S., McCarthy K., Le T., Weiner L. P. Patterns of cytokine secretion by autoreactive proteolipid protein-specific T cell clones during the course of multiple sclerosis. J Immunol. 1995 Mar 15;154(6):2959–2968. [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Mosmann T. R., Vitetta E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988 Aug 1;168(2):543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Katsikis P. D., Cohen S. B., Londei M., Feldmann M. Are CD4+ Th1 cells pro-inflammatory or anti-inflammatory? The ratio of IL-10 to IFN-gamma or IL-2 determines their function. Int Immunol. 1995 Aug;7(8):1287–1294. doi: 10.1093/intimm/7.8.1287. [DOI] [PubMed] [Google Scholar]
- King C. L., Stupi R. J., Craighead N., June C. H., Thyphronitis G. CD28 activation promotes Th2 subset differentiation by human CD4+ cells. Eur J Immunol. 1995 Feb;25(2):587–595. doi: 10.1002/eji.1830250242. [DOI] [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., de Kuiper R., Daha M. R., Breedveld F. C. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992 May;35(5):603–610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Mocci S., Coffman R. L. Induction of a Th2 population from a polarized Leishmania-specific Th1 population by in vitro culture with IL-4. J Immunol. 1995 Apr 15;154(8):3779–3787. [PubMed] [Google Scholar]
- Mullins R. J., Cohen S. B., Webb L. M., Chernajovsky Y., Dayan C. M., Londei M., Feldmann M. Identification of thyroid stimulating hormone receptor-specific T cells in Graves' disease thyroid using autoantigen-transfected Epstein-Barr virus-transformed B cell lines. J Clin Invest. 1995 Jul;96(1):30–37. doi: 10.1172/JCI118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliard X., de Vries J. E., Spits H. Comparison of lymphokine secretion and responsiveness of human T cell clones isolated in IL-4 and in IL-2. Cell Immunol. 1991 Jul;135(2):383–393. doi: 10.1016/0008-8749(91)90283-h. [DOI] [PubMed] [Google Scholar]
- Pernis A., Gupta S., Gollob K. J., Garfein E., Coffman R. L., Schindler C., Rothman P. Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science. 1995 Jul 14;269(5221):245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- Quayle A. J., Chomarat P., Miossec P., Kjeldsen-Kragh J., Førre O., Natvig J. B. Rheumatoid inflammatory T-cell clones express mostly Th1 but also Th2 and mixed (Th0-like) cytokine patterns. Scand J Immunol. 1993 Jul;38(1):75–82. doi: 10.1111/j.1365-3083.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Yssel H., De Waal Malefyt R., Roncarolo M. G., Abrams J. S., Lahesmaa R., Spits H., de Vries J. E. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992 Oct 1;149(7):2378–2384. [PubMed] [Google Scholar]