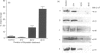Abstract
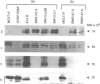
Lymphocyte migration into inflammatory sites involves a change from a spherical, non-motile phenotype to an irregular, constantly shape-changing, motile phenotype. We have previously shown that lymphocytes are maintained in the non-motile state by the constitutive activity of protein kinase C (PKC). In this paper we have attempted to identify the PKC isotype which regulates these morphological changes by three different approaches. (a) Motile and non-motile T-cell lines were compared for expression of the alpha, beta I, beta II, gamma, delta, epsilon, eta, zeta and theta isotypes by Western blotting. There was no obvious correlation of isotype expression with motility. (b) Two different PKC inhibitors, one specific for classical isotypes, Go6976 and the other GF109203X, which inhibits both classical and non-classical isotypes were compared for induction of motility in non-motile lymphocytes. Only GF109203X induced motility implying that a non-classical isotype is involved. (c) Non-motile lymphocytes were chronically treated with the PKC activator bryostatin and the time courses of induction of motility and downregulation of PKC isotypes were compared. Induction of motility correlated better with downregulation of epsilon, eta and theta than with alpha or beta. It is concluded that the data fit best with the involvement of a non-classical PKC isotype in regulating lymphocyte motility although no association with a particular isotype was found.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuadrado A., Issing W., Fleming T. P., Molloy C. J. Uneven distribution of protein kinase C-alpha and -beta isozymes in human sarcomas and carcinomas. J Cell Physiol. 1994 Jun;159(3):434–440. doi: 10.1002/jcp.1041590307. [DOI] [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J. Protein kinase C--a question of specificity. Trends Biochem Sci. 1994 Feb;19(2):73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Gamard C. J., Blobe G. C., Hannun Y. A., Obeid L. M. Specific role for protein kinase C beta in cell differentiation. Cell Growth Differ. 1994 Apr;5(4):405–409. [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucas S., Marais R., Graves J. D., Alexander D., Parker P., Cantrell D. A. Heterogeneity of protein kinase C expression and regulation in T lymphocytes. FEBS Lett. 1990 Jan 15;260(1):53–56. doi: 10.1016/0014-5793(90)80064-p. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993 May 5;268(13):9194–9197. [PubMed] [Google Scholar]
- McGlynn E., Liebetanz J., Reutener S., Wood J., Lydon N. B., Hofstetter H., Vanek M., Meyer T., Fabbro D. Expression and partial characterization of rat protein kinase C-delta and protein kinase C-zeta in insect cells using recombinant baculovirus. J Cell Biochem. 1992 Jul;49(3):239–250. doi: 10.1002/jcb.240490306. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern C., Wilkinson P. C., Thorp K. M., Henderson L. K., Nemec M., Matthews N. Inhibition of protein kinase C results in a switch from a non-motile to a motile phenotype in diverse human lymphocyte populations. Immunology. 1995 Feb;84(2):326–332. [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Parker P. J. Protein kinase C. Pharmacol Ther. 1991;51(1):71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- Thorp K. M., Southern C., Matthews N. Effect of serine/threonine kinase inhibitors on motility of human lymphocytes and U937 cells. Immunology. 1994 Apr;81(4):546–550. [PMC free article] [PubMed] [Google Scholar]
- Trachsel S., Keller H. U. Selective responses (actin polymerization, shape changes, locomotion, pinocytosis) to the PKC inhibitor Ro 31-8220 suggest that PKC discriminately regulates functions of human blood lymphocytes. J Leukoc Biol. 1995 Apr;57(4):587–591. doi: 10.1002/jlb.57.4.587. [DOI] [PubMed] [Google Scholar]
- Verschueren H., Van der Taelen I., Dewit J., De Braekeleer J., De Baetselier P. Metastatic competence of BW5147 T-lymphoma cell lines is correlated with in vitro invasiveness, motility and F-actin content. J Leukoc Biol. 1994 Apr;55(4):552–556. doi: 10.1002/jlb.55.4.552. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Liew F. Y. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995 Mar 1;181(3):1255–1259. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]