Abstract
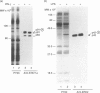
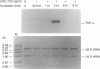
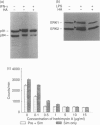
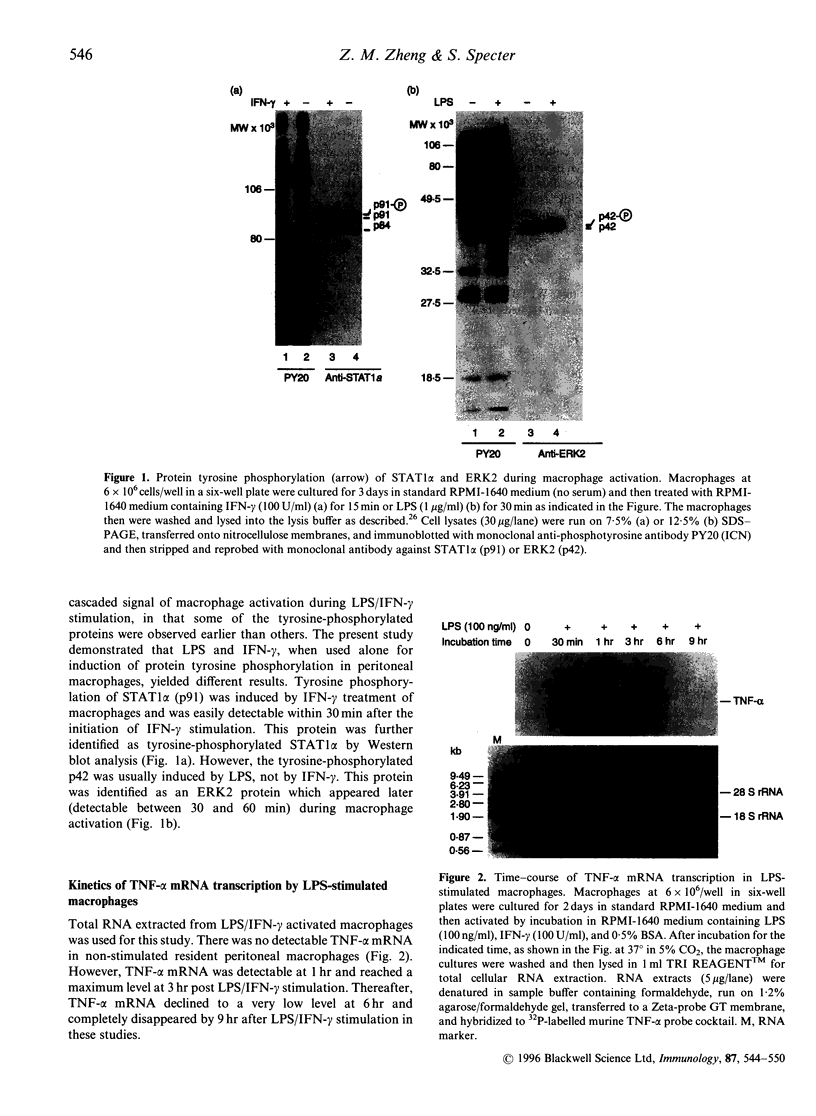
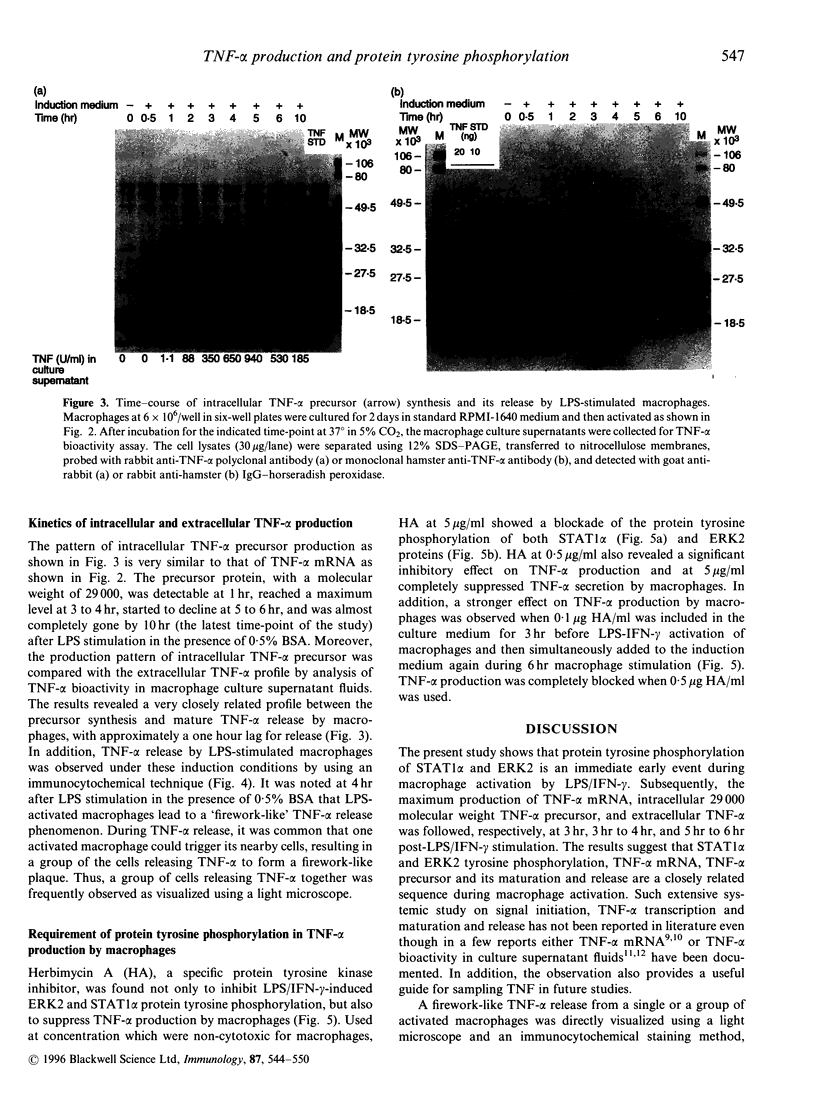
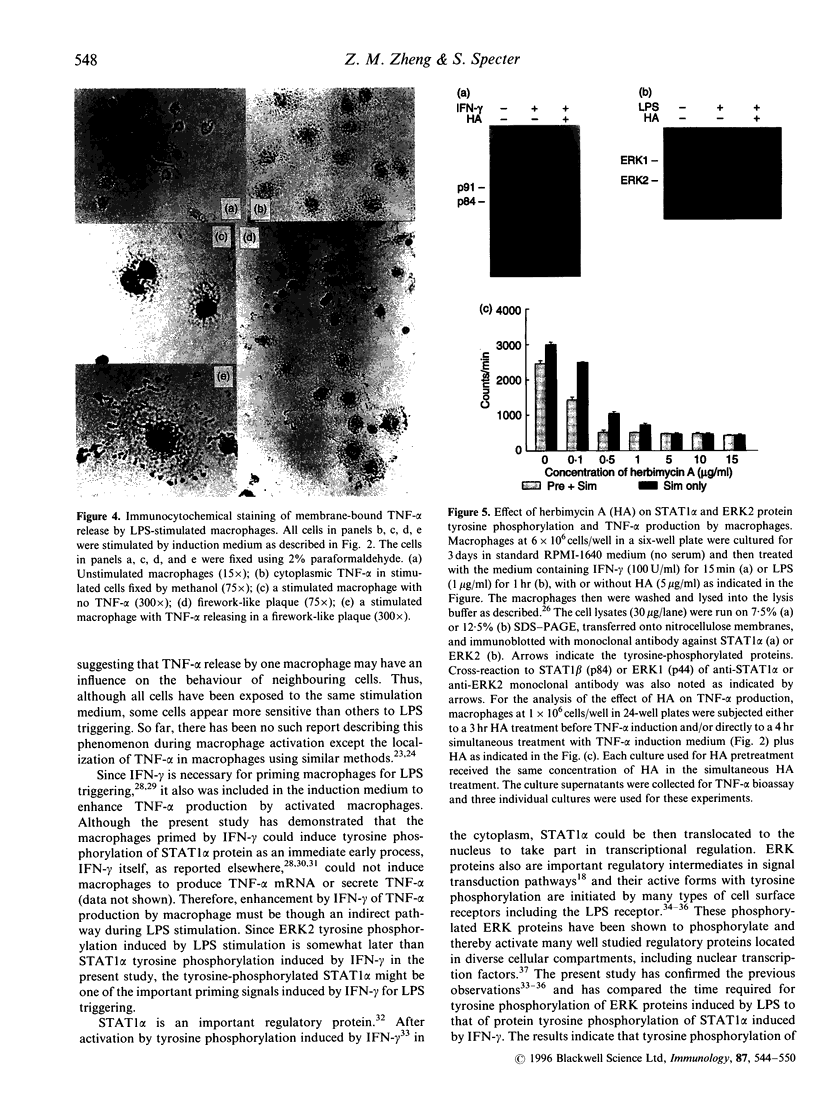
Tumour necrosis factor-alpha (TNF-alpha), an important mediator in both immune and inflammation responses, is one of the major cytokines released by activated macrophages. The present study shows that, during macrophage activation, protein tyrosine phosphorylation of STAT1 alpha and ERK2 occurred as an immediate early signal, whereas maximum TNF-alpha mRNA transcription appeared at 3 hr, precursor TNF-alpha formation at 3 to 4 hr, and TNF-alpha release at 5 to 6 hr after stimulation of an RPMI-1640-based induction medium containing lipopolysaccharide (100 ng/ml), interferon-gamma (100 U/ml), and 0.5% bovine serum albumin. Herbimycin A, a tyrosine kinase inhibitor, suppresses protein tyrosine phosphorylation of STAT1 alpha and ERK2 and also blocks TNF-alpha production by resident peritoneal macrophages from BALB/c mice, suggesting a possible association between protein tyrosine phosphorylation of STAT1 alpha and ERK2 and macrophage activation resulting in TNF-alpha production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Khabar K. S., Armstrong J. A., Ho M. Type I interferons (IFN-alpha and -beta) suppress cytotoxin (tumor necrosis factor-alpha and lymphotoxin) production by mitogen-stimulated human peripheral blood mononuclear cell. J Leukoc Biol. 1992 Aug;52(2):165–172. doi: 10.1002/jlb.52.2.165. [DOI] [PubMed] [Google Scholar]
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Barak M., Gruener N. Neopterin augmentation of tumor necrosis factor production. Immunol Lett. 1991 Sep;30(1):101–106. doi: 10.1016/0165-2478(91)90096-s. [DOI] [PubMed] [Google Scholar]
- Blasi E., Pitzurra L., Puliti M., Bartoli A., Bistoni F. Candida albicans hyphal form enhances tumor necrosis factor mRNA levels and protein secretion in murine ANA-1 macrophages. Cell Immunol. 1992 Jun;142(1):137–144. doi: 10.1016/0008-8749(92)90275-t. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C., Ding A. Taxol, a microtubule-stabilizing antineoplastic agent, induces expression of tumor necrosis factor alpha and interleukin-1 in macrophages. J Leukoc Biol. 1992 Jul;52(1):119–121. doi: 10.1002/jlb.52.1.119. [DOI] [PubMed] [Google Scholar]
- Brown A. R., Fishman M. Tumor necrosis factor-alpha analyzed within individual macrophages by combined immunocytochemistry and computer-aided image analysis. Cell Immunol. 1990 Oct 15;130(2):352–363. doi: 10.1016/0008-8749(90)90278-y. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Remick D. G., Shmyr-Forsch C., Beals T. F., Kunkel S. L. Immunohistochemical demonstration of cytoplasmic and membrane-associated tumor necrosis factor in murine macrophages. Am J Pathol. 1988 Dec;133(3):564–572. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Ding A. H., Porteu F., Sanchez E., Nathan C. F. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990 Apr 20;248(4953):370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- Dong Z., Qi X., Fidler I. J. Tyrosine phosphorylation of mitogen-activated protein kinases is necessary for activation of murine macrophages by natural and synthetic bacterial products. J Exp Med. 1993 Apr 1;177(4):1071–1077. doi: 10.1084/jem.177.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari M. K., Nguyen D. T., Kunkel S. L., Remick D. G. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol Invest. 1990 Feb;19(1):69–79. doi: 10.3109/08820139009042026. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Gifford G. E., Flick D. A., AbdAllah N. A., Fisch H. Production of a cytotoxin from phorbol myristate acetate-treated human promyelocytes. J Natl Cancer Inst. 1984 Jul;73(1):69–73. [PubMed] [Google Scholar]
- Hill C. S., Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995 Jan 27;80(2):199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Manthey C. L., Perera P. Y., Salkowski C. A., Vogel S. N. Taxol provides a second signal for murine macrophage tumoricidal activity. J Immunol. 1994 Jan 15;152(2):825–831. [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Schreiber R. D., Altman A., Katz D. H. Macrophage activation: priming activity from a T-cell hybridoma is attributable to interferon-gamma. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3782–3786. doi: 10.1073/pnas.80.12.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Shivers S. C., Newton C., Friedman H., Klein T. W. delta 9-Tetrahydrocannabinol (THC) modulates IL-1 bioactivity in human monocyte/macrophage cell lines. Life Sci. 1994;54(17):1281–1289. doi: 10.1016/0024-3205(94)00856-6. [DOI] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Sprecher E., Becker Y. Detection of IL-1 beta, TNF-alpha, and IL-6 gene transcription by the polymerase chain reaction in keratinocytes, Langerhans cells and peritoneal exudate cells during infection with herpes simplex virus-1. Arch Virol. 1992;126(1-4):253–269. doi: 10.1007/BF01309699. [DOI] [PubMed] [Google Scholar]
- Tachibana K., Chen G. J., Huang D. S., Scuderi P., Watson R. R. Production of tumor necrosis factor alpha by resident and activated murine macrophages. J Leukoc Biol. 1992 Mar;51(3):251–255. doi: 10.1002/jlb.51.3.251. [DOI] [PubMed] [Google Scholar]
- Tachibana K., Chen G. J., Huang D. S., Scuderi P., Watson R. R. Production of tumor necrosis factor alpha by resident and activated murine macrophages. J Leukoc Biol. 1992 Mar;51(3):251–255. doi: 10.1002/jlb.51.3.251. [DOI] [PubMed] [Google Scholar]
- Tebo J. M., Hamilton T. A. Okadaic acid stimulates inflammatory cytokine gene transcription in murine peritoneal macrophages. Cell Immunol. 1994 Feb;153(2):479–491. doi: 10.1006/cimm.1994.1044. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H. Use and selectivity of herbimycin A as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:370–379. doi: 10.1016/0076-6879(91)01033-x. [DOI] [PubMed] [Google Scholar]
- Watzl B., Scuderi P., Watson R. R. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. Int J Immunopharmacol. 1991;13(8):1091–1097. doi: 10.1016/0192-0561(91)90160-9. [DOI] [PubMed] [Google Scholar]
- Weinstein S. L., June C. H., DeFranco A. L. Lipopolysaccharide-induced protein tyrosine phosphorylation in human macrophages is mediated by CD14. J Immunol. 1993 Oct 1;151(7):3829–3838. [PubMed] [Google Scholar]
- Weinstein S. L., Sanghera J. S., Lemke K., DeFranco A. L., Pelech S. L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992 Jul 25;267(21):14955–14962. [PubMed] [Google Scholar]
- Wollenberg G. K., DeForge L. E., Bolgos G., Remick D. G. Differential expression of tumor necrosis factor and interleukin-6 by peritoneal macrophages in vivo and in culture. Am J Pathol. 1993 Oct;143(4):1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., Koerner T. J., Adams D. O. Gene regulation in macrophage activation: differential regulation of genes encoding for tumor necrosis factor, interleukin-1, JE, and KC by interferon-gamma and lipopolysaccharide. J Leukoc Biol. 1990 Nov;48(5):412–419. doi: 10.1002/jlb.48.5.412. [DOI] [PubMed] [Google Scholar]
- Zheng Z. M., Specter S. C., Lancz G. Bovine serum albumin preparations enhance in vitro production of tumor necrosis factor alpha by murine macrophages. Immunol Invest. 1995 Aug;24(5):737–756. doi: 10.3109/08820139509060702. [DOI] [PubMed] [Google Scholar]
- Zheng Z. M., Specter S., Friedman H. Inhibition by delta-9-tetrahydrocannabinol of tumor necrosis factor alpha production by mouse and human macrophages. Int J Immunopharmacol. 1992 Nov;14(8):1445–1452. doi: 10.1016/0192-0561(92)90017-f. [DOI] [PubMed] [Google Scholar]
- Zheng Z. M., Specter S. Suppression by delta-9-tetrahydrocannabinol of lipopolysaccharide-induced and intrinsic tyrosine phosphorylation and protein expression in mouse peritoneal macrophages. Biochem Pharmacol. 1994 Jun 15;47(12):2243–2252. doi: 10.1016/0006-2952(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Evans G. F., Butler L. D. Endotoxin tolerance: independent regulation of interleukin-1 and tumor necrosis factor expression. Infect Immun. 1991 Aug;59(8):2774–2780. doi: 10.1128/iai.59.8.2774-2780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]