Abstract
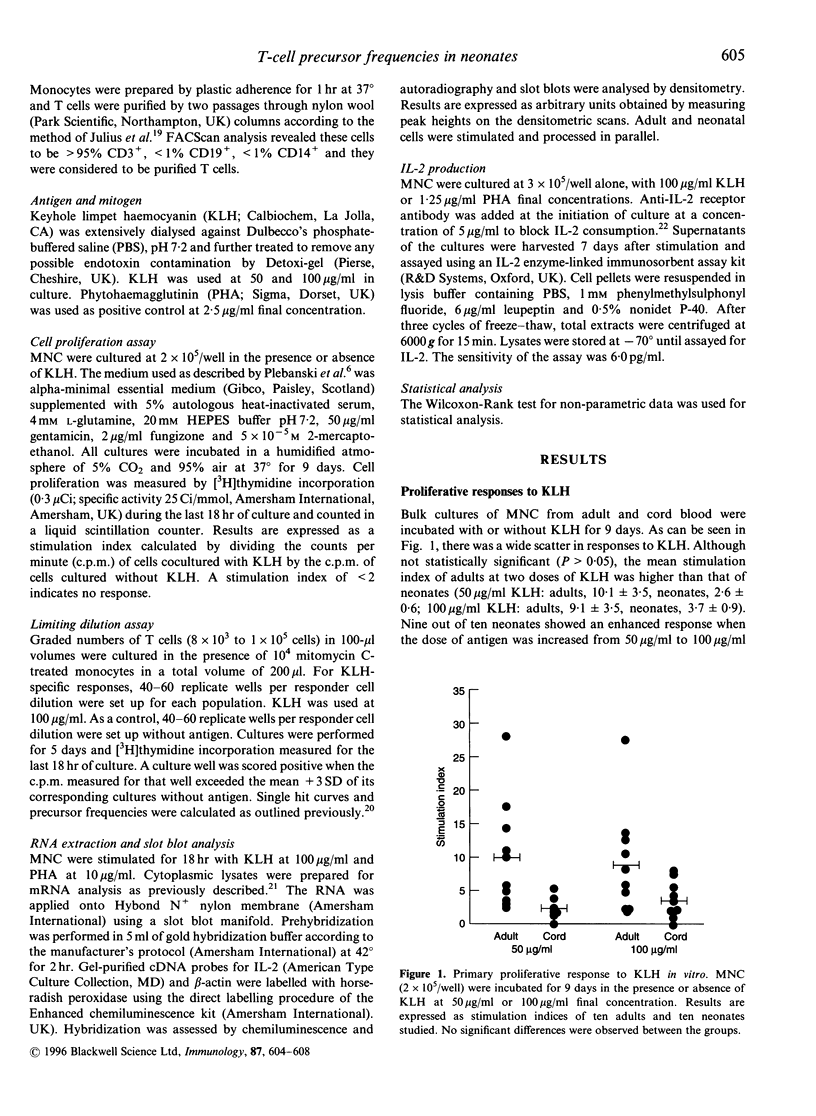
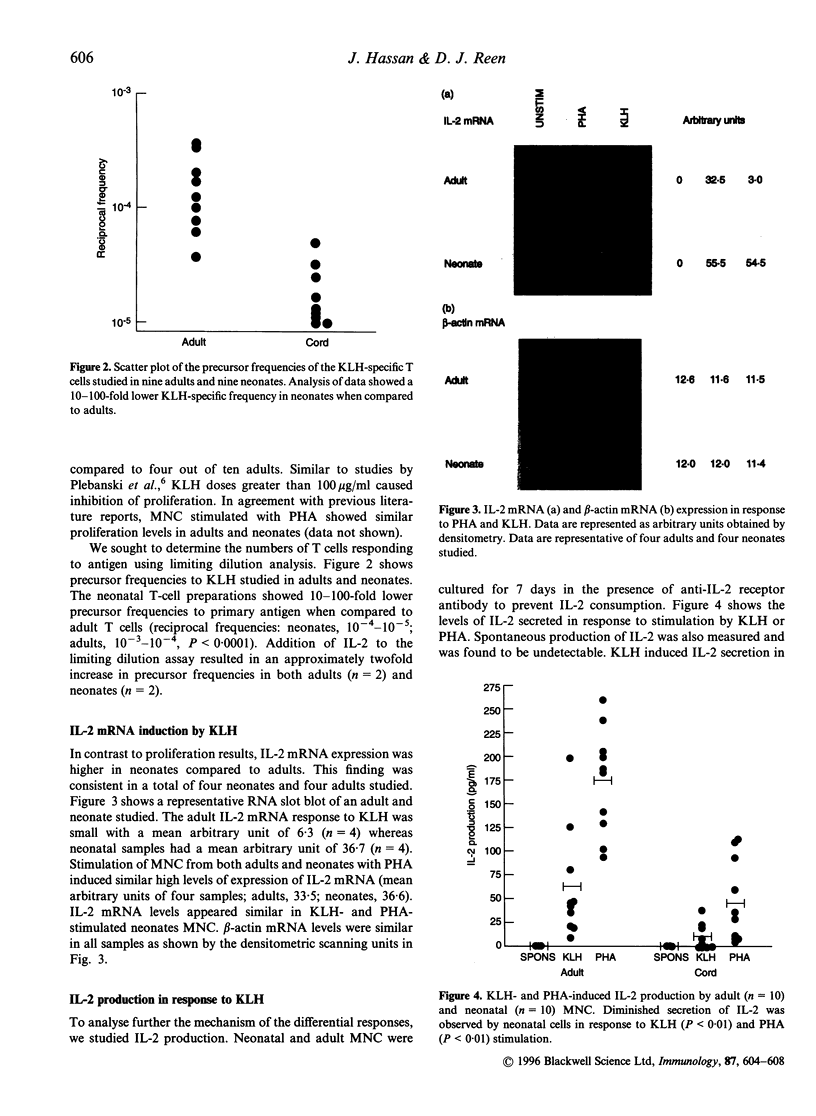
Clinical evidence has indicated that the neonatal cell-mediated immune response to primary infection is delayed when compared to that of adults with the same primary infection. The mechanisms regulating the development of antigen-specific T-cell immunity in neonates remain to be elucidated. We examined the primary immune response to the non-recall antigen, keyhole limpet haemocyanin (KLH) in adults and neonates in vitro. We report here that conventional bulk culture methods show reduced proliferative responses in neonates although statistical significance was not achieved. Using limiting dilution analysis, the frequencies of KLH-specific T lymphocytes were 10-100-fold lower in neonates when compared to adults. Interleukin-2 (IL-2) production was significantly lower in the supernatants of neonatal mononuclear cells (MNC) stimulated with KLH when compared to adults. Addition of exogenous IL-2 increased precursor frequencies twofold in both adult and newborn cultures. In contrast to the secreted IL-2 levels, IL-2 mRNA expression was higher in antigen-stimulated neonatal MNC preparations, even though proliferation was lower. These observations indicate differential in vitro responsiveness in neonates and adults to primary antigenic challenge. Since no IL-2 was detected in cell lysates, the presence of high levels of IL-2 mRNA and low IL-2 production suggests inability by neonatal MNC to translate IL-2. This deficiency in IL-2 production may explain the reduced precursor frequencies, suggesting failure to recruit T lymphocytes in order to expand the KLH-specific T-cell response. These observations are important for the understanding of the development of primary immune responses and immunological maturation in neonates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Salmon M., Ivory K., Taki S., Pilling D., Janossy G. Human CD4+CD45R0+ and CD4+CD45RA+ T cells synergize in response to alloantigens. Eur J Immunol. 1991 Oct;21(10):2517–2522. doi: 10.1002/eji.1830211031. [DOI] [PubMed] [Google Scholar]
- Bryson Y. J., Winter H. S., Gard S. E., Fischer T. J., Stiehm E. R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980 Sep 15;55(1):191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Chinn A., Cosyns M., Hayward A. R. T cell proliferative response to interleukin 2: different frequency of responders among CD45R0 and CD45RA subsets. Cell Immunol. 1990 Nov;131(1):132–139. doi: 10.1016/0008-8749(90)90240-r. [DOI] [PubMed] [Google Scholar]
- Clerici M., DePalma L., Roilides E., Baker R., Shearer G. M. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J Clin Invest. 1993 Jun;91(6):2829–2836. doi: 10.1172/JCI116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax C. A., Borzy M. S. Interleukin 2 production, proliferative response, and receptor expression by cord blood mononuclear cells. J Clin Lab Immunol. 1988 Oct;27(2):63–67. [PubMed] [Google Scholar]
- Hannet I., Erkeller-Yuksel F., Lydyard P., Deneys V., DeBruyère M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992 Jun;13(6):215–218. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- Hassan J., Reen D. J. Interleukin-1 augments the diminished interleukin-2 mRNA expression and proliferative response of neonatal T lymphocytes to anti-CD2 antibodies. Scand J Immunol. 1994 Jun;39(6):597–601. doi: 10.1111/j.1365-3083.1994.tb03418.x. [DOI] [PubMed] [Google Scholar]
- Hassan J., Reen D. J. Neonatal CD4+ CD45RA+ T cells: precursors of adult CD4+ CD45RA+ T cells? Res Immunol. 1993 Feb;144(2):87–92. doi: 10.1016/0923-2494(93)80064-6. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Herberger M., Saunders D. Herpes simplex virus-stimulated gamma-interferon production by newborn mononuclear cells. Pediatr Res. 1986 May;20(5):398–401. doi: 10.1203/00006450-198605000-00004. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Lewis D. B., Larsen A., Wilson C. B. Reduced interferon-gamma mRNA levels in human neonates. Evidence for an intrinsic T cell deficiency independent of other genes involved in T cell activation. J Exp Med. 1986 Apr 1;163(4):1018–1023. doi: 10.1084/jem.163.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Meyer J., English B. K., Kahn S. J., Wilson C. B. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Invest. 1991 Jan;87(1):194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebanski M., Saunders M., Burtles S. S., Crowe S., Hooper D. C. Primary and secondary human in vitro T-cell responses to soluble antigens are mediated by subsets bearing different CD45 isoforms. Immunology. 1992 Jan;75(1):86–91. [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Splawski J. B., Jelinek D. F., Lipsky P. E. Delineation of the functional capacity of human neonatal lymphocytes. J Clin Invest. 1991 Feb;87(2):545–553. doi: 10.1172/JCI115029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L., Arenzana-Seisdedos F., Rothhut B., Huerta J. M., Russo-Marie F., Fiers W. Defective IFN-gamma production in the human neonate. II. Role of increased sensitivity to the suppressive effects of prostaglandin E. J Immunol. 1985 Jan;134(1):172–176. [PubMed] [Google Scholar]
- Watson W., Oen K., Ramdahin R., Harman C. Immunoglobulin and cytokine production by neonatal lymphocytes. Clin Exp Immunol. 1991 Jan;83(1):169–174. doi: 10.1111/j.1365-2249.1991.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B. Immunologic basis for increased susceptibility of the neonate to infection. J Pediatr. 1986 Jan;108(1):1–12. doi: 10.1016/s0022-3476(86)80761-2. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Westall J., Johnston L., Lewis D. B., Dower S. K., Alpert A. R. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J Clin Invest. 1986 Mar;77(3):860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlabinger G. J., Mannhalter J. W., Eibl M. M. Cord blood macrophages present bacterial antigen (Escherichia coli) to paternal T cells. Clin Immunol Immunopathol. 1983 Sep;28(3):405–412. doi: 10.1016/0090-1229(83)90107-1. [DOI] [PubMed] [Google Scholar]
- van Tol M. J., Zijlstra J., Thomas C. M., Zegers B. J., Ballieux R. E. Distinct role of neonatal and adult monocytes in the regulation of the in vitro antigen-induced plaque-forming cell response in man. J Immunol. 1984 Oct;133(4):1902–1908. [PubMed] [Google Scholar]