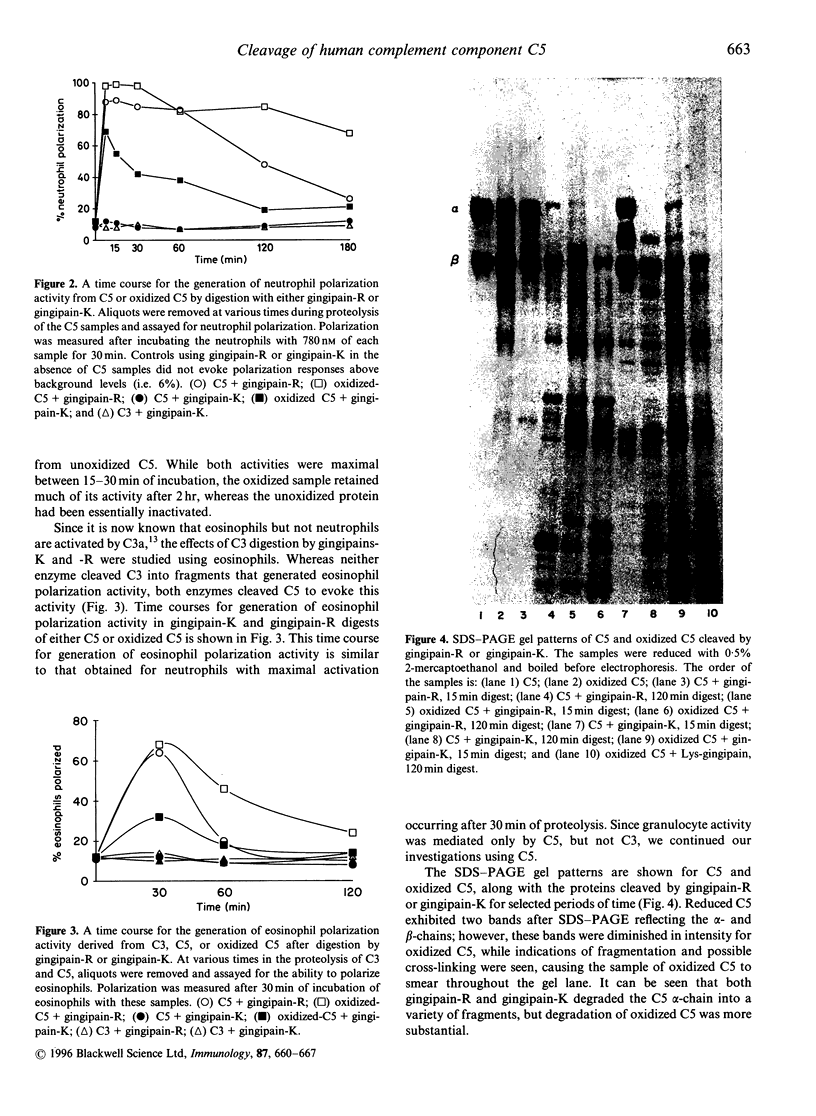
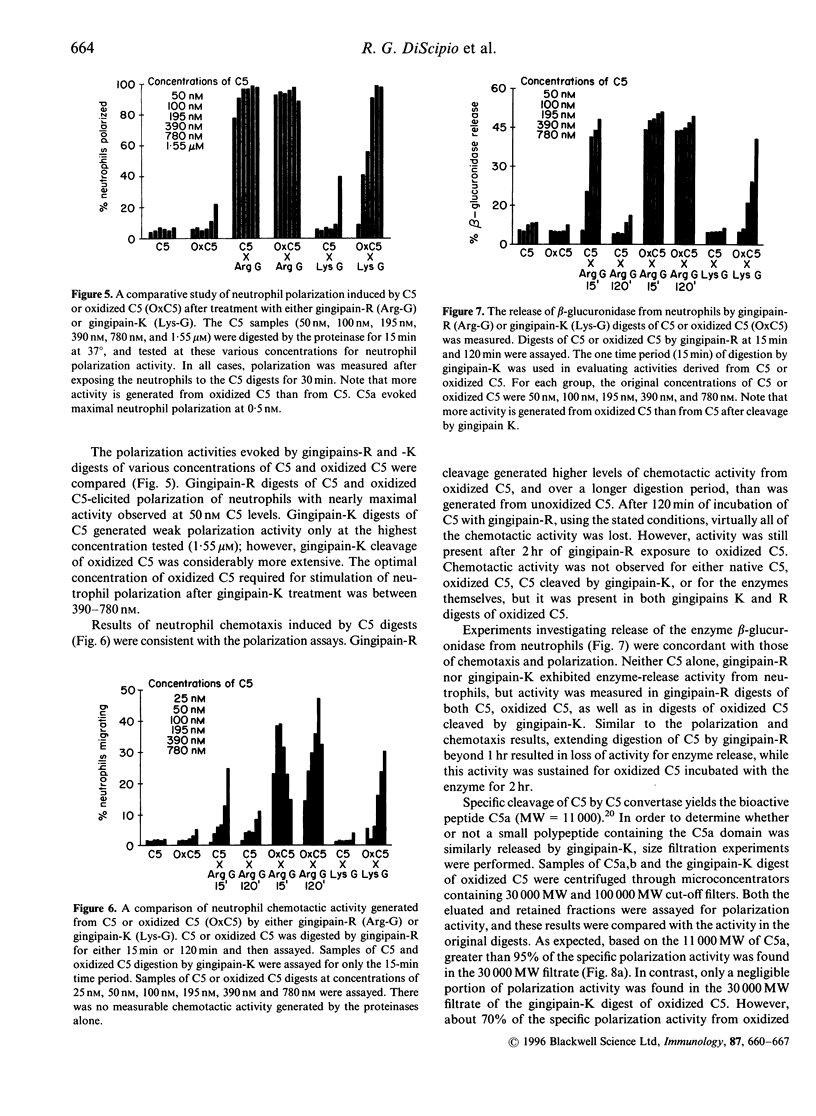
Abstract
Since severe periodontitis is characterized by an acute inflammatory response with cellular infiltration and microbial overgrowth, plasma proteins could be exposed to both proteinases and oxidants released from the granulocytes, as well as to proteinases from the microorganisms. When human complement component C5 was digested by cysteine proteinases (i.e. gingipain-R and gingipain-K) from Porphyromonas gingivalis, limited cleavage of the C5 molecule was observed. If C5 was first oxidized by hydroxyl radicals, these gingipains converted modified C5 to fragments that exhibited significantly greater pro-inflammatory activity than did digests of unmodified C5. After cleavage of oxidized C5 by gingipain-R, the digest exhibited measurably greater neutrophil enzyme release and chemotaxis of human polymorphonuclear leukocytes (PMNs) compared with the activities of unoxidized C5 digests. Gingipain-K generates virtually no polarization or chemotactic activity of human PMNs from C5, nor is enzyme release stimulated by these C5 digests. However, when oxidized C5 was digested by gingipain-K, human PMNs were stimulated for polarization, chemotaxis and enzyme release indicating that an active fragment had been generated. Proteolysis of oxidized C5 evokes greater neutrophil activation than does proteolysis of unoxidized protein, a fact which supports the hypothesis that oxidation and proteolysis may be coupled to enhance the destructive effects of the inflammatory process. These results, in which digests of both oxidized and unmodified complement component C5 were evaluated, support the general concept that oxidation and proteolysis may participate cooperatively in amplifying both the severity and duration of the inflammatory reaction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Z., Potempa J., Polanowski A., Wikstrom M., Travis J. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. J Biol Chem. 1992 Sep 15;267(26):18896–18901. [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffern P. J., Pfeifer P. H., Ember J. A., Hugli T. E. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995 Jun 1;181(6):2119–2127. doi: 10.1084/jem.181.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E., Lin S. W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987 Jul 15;262(20):9902–9907. [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J Biol Chem. 1987 Jul 15;262(20):9908–9913. [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- Dean R. T., Gieseg S., Davies M. J. Reactive species and their accumulation on radical-damaged proteins. Trends Biochem Sci. 1993 Nov;18(11):437–441. doi: 10.1016/0968-0004(93)90145-d. [DOI] [PubMed] [Google Scholar]
- DiScipio R. G., Smith C. A., Muller-Eberhard H. J., Hugli T. E. The activation of human complement component C5 by a fluid phase C5 convertase. J Biol Chem. 1983 Sep 10;258(17):10629–10636. [PubMed] [Google Scholar]
- DiScipio R. G., Sweeney S. P. The fractionation of human plasma proteins. II. The purification of human complement proteins C3, C3u, and C5 by application of affinity chromatography. Protein Expr Purif. 1994 Apr;5(2):170–177. doi: 10.1006/prep.1994.1027. [DOI] [PubMed] [Google Scholar]
- Elsner J., Oppermann M., Czech W., Dobos G., Schöpf E., Norgauer J., Kapp A. C3a activates reactive oxygen radical species production and intracellular calcium transients in human eosinophils. Eur J Immunol. 1994 Mar;24(3):518–522. doi: 10.1002/eji.1830240304. [DOI] [PubMed] [Google Scholar]
- Ember J. A., Sanderson S. D., Taylor S. M., Kawahara M., Hugli T. E. Biologic activity of synthetic analogues of C5a anaphylatoxin. J Immunol. 1992 May 15;148(10):3165–3173. [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Grenier D., Chao G., McBride B. C. Characterization of sodium dodecyl sulfate-stable Bacteroides gingivalis proteases by polyacrylamide gel electrophoresis. Infect Immun. 1989 Jan;57(1):95–99. doi: 10.1128/iai.57.1.95-99.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Selected characteristics of pathogenic and nonpathogenic strains of Bacteroides gingivalis. J Clin Microbiol. 1987 Apr;25(4):738–740. doi: 10.1128/jcm.25.4.738-740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P. D., McKee A. S., McDermid A. S., Dowsett A. B. Ultrastructure and enzyme activities of a virulent and an avirulent variant of Bacteroides gingivalis W50. FEMS Microbiol Lett. 1989 May;50(1-2):181–185. doi: 10.1016/0378-1097(89)90482-5. [DOI] [PubMed] [Google Scholar]
- Morgan E. L., Ember J. A., Sanderson S. D., Scholz W., Buchner R., Ye R. D., Hugli T. E. Anti-C5a receptor antibodies. Characterization of neutralizing antibodies specific for a peptide, C5aR-(9-29), derived from the predicted amino-terminal sequence of the human C5a receptor. J Immunol. 1993 Jul 1;151(1):377–388. [PubMed] [Google Scholar]
- Pike R., McGraw W., Potempa J., Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994 Jan 7;269(1):406–411. [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Sugita N., Suzuki T., Yoshie H., Yoshida N., Adachi M., Hara K. Differential expression of CR3, Fc epsilon RII and Fc gamma RIII on polymorphonuclear leukocytes in gingival crevicular fluid. J Periodontal Res. 1993 Sep;28(5):363–372. doi: 10.1111/j.1600-0765.1993.tb01080.x. [DOI] [PubMed] [Google Scholar]
- Uchida K., Kato Y., Kawakishi S. A novel mechanism for oxidative cleavage of prolyl peptides induced by the hydroxyl radical. Biochem Biophys Res Commun. 1990 May 31;169(1):265–271. doi: 10.1016/0006-291x(90)91463-3. [DOI] [PubMed] [Google Scholar]
- Vogi W., Nolte R., Brunahl D. Binding of iron to the 5th component of human complement directs oxygen radical-mediated conversion to specific sites and causes nonenzymic activation. Complement Inflamm. 1991;8(5-6):313–319. doi: 10.1159/000463202. [DOI] [PubMed] [Google Scholar]
- Vogt W., Damerau B., von Zabern I., Nolte R., Brunahl D. Non-enzymic activation of the fifth component of human complement, by oxygen radicals. Some properties of the activation product, C5b-like C5. Mol Immunol. 1989 Dec;26(12):1133–1142. doi: 10.1016/0161-5890(89)90057-6. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wetsel R. A., Kolb W. P. Complement-independent activation of the fifth component (C5) of human complement: limited trypsin digestion resulting in the expression of biological activity. J Immunol. 1982 May;128(5):2209–2216. [PubMed] [Google Scholar]
- White D., Mayrand D. Association of oral Bacteroides with gingivitis and adult periodontitis. J Periodontal Res. 1981 May;16(3):259–265. doi: 10.1111/j.1600-0765.1981.tb00974.x. [DOI] [PubMed] [Google Scholar]
- von Zabern W. V., Hesse D., Nolte R., Haller Y. Generation of an activated form of human C5 (C5b-like C5) by oxygen radicals. Immunol Lett. 1987 Feb;14(3):209–215. doi: 10.1016/0165-2478(87)90103-9. [DOI] [PubMed] [Google Scholar]