Abstract
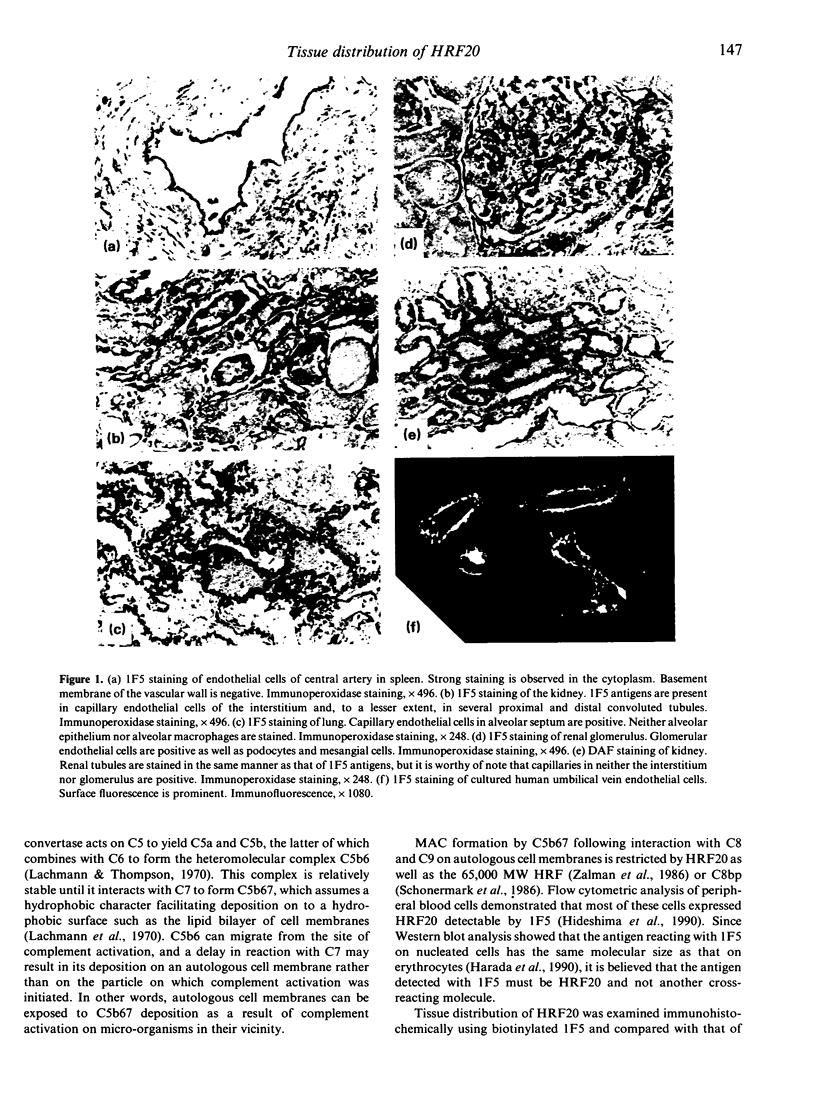
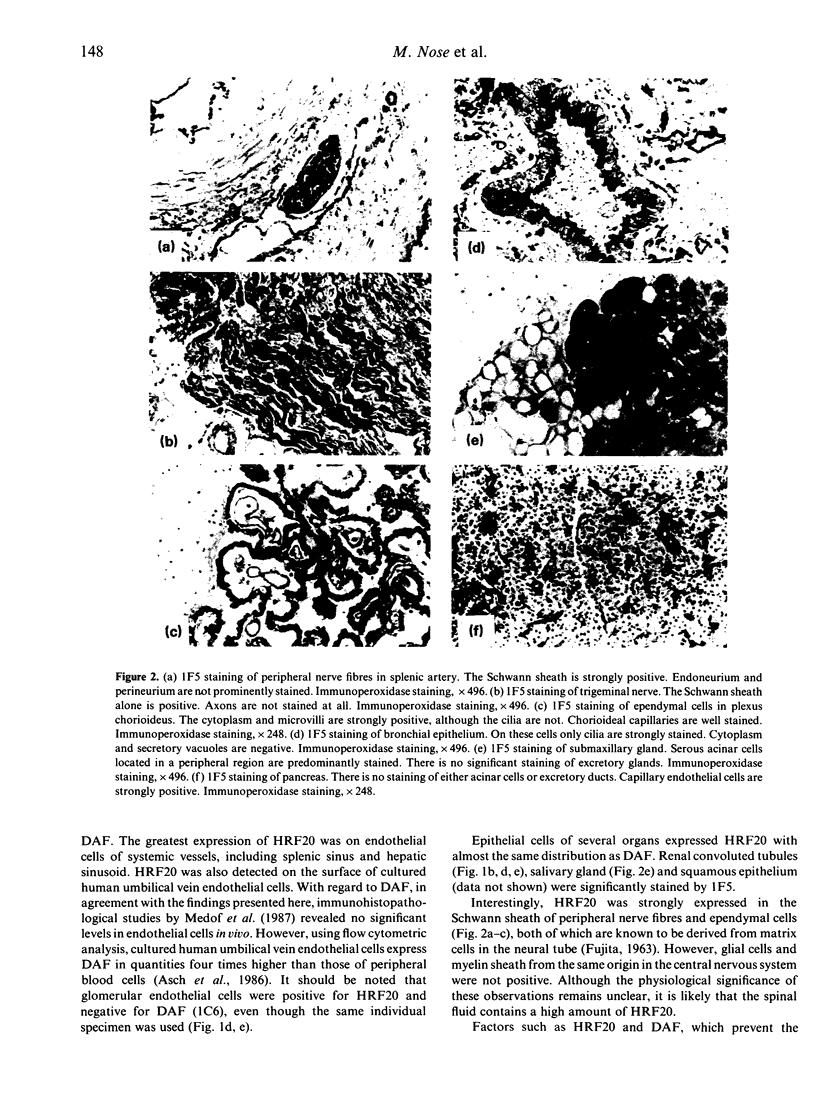
A 20,000 molecular weight (MW) homologous restriction factor (HRF20), detected by 1F5 monoclonal antibody (mAb), is present on blood cell surfaces and inhibits the terminal stage of the formation of membrane attack complexes by homologous complement activation. The tissue distribution of HRF20 was studied by immunohistochemical analysis using 1F5. HRF20 was predominantly expressed on endothelial cells of systemic arteries, veins and capillaries, as well as on the surface of cultured human umbilical vein endothelial cells. HRF20 was also detected, to a lesser extent, on the Schwann sheath of peripheral nerve fibres, ependymal cells and certain epithelial cells such as acinar cells of the salivary gland, bronchial epithelium, renal tubules and squamous epithelium. The distribution pattern of HRF20 differed somewhat from that of decay-accelerating factor (DAF), which is another membrane inhibitor of homologous complement activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Kinoshita T., Jaffe E. A., Nussenzweig V. Decay-accelerating factor is present on cultured human umbilical vein endothelial cells. J Exp Med. 1986 Jan 1;163(1):221–226. doi: 10.1084/jem.163.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJITA S. The matrix cell and cytogenesis in the developing central nervous system. J Comp Neurol. 1963 Feb;120:37–42. doi: 10.1002/cne.901200104. [DOI] [PubMed] [Google Scholar]
- Harada R., Okada N., Fujita T., Okada H. Purification of 1F5 antigen that prevents complement attack on homologous cell membranes. J Immunol. 1990 Mar 1;144(5):1823–1828. [PubMed] [Google Scholar]
- Hideshima T., Okada N., Okada H. Expression of HRF20, a regulatory molecule of complement activation, on peripheral blood mononuclear cells. Immunology. 1990 Mar;69(3):396–401. [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. M. Inhibition of complement by a substance isolated from human erythrocytes. I. Extraction from human erythrocyte stromata. Immunochemistry. 1969 May;6(3):391–403. doi: 10.1016/0019-2791(69)90296-1. [DOI] [PubMed] [Google Scholar]
- Holguin M. H., Fredrick L. R., Bernshaw N. J., Wilcox L. A., Parker C. J. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989 Jul;84(1):7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Munn E. A., Weissmanng Complement-mediated lysis of liposomes produced by the reactive lysis procedure. Immunology. 1970 Dec;19(6):983–986. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Thompson R. A. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970 Apr 1;131(4):643–657. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Okada H., Nagami Y., Takahashi K., Okada N., Hideshima T., Takizawa H., Kondo J. 20 KDa homologous restriction factor of complement resembles T cell activating protein. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1553–1559. doi: 10.1016/0006-291x(89)90852-8. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1(2):205–208. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada R., Fujita T., Okada H. Monoclonal antibodies capable of causing hemolysis of neuraminidase-treated human erythrocytes by homologous complement. J Immunol. 1989 Oct 1;143(7):2262–2266. [PubMed] [Google Scholar]
- Schönermark S., Rauterberg E. W., Shin M. L., Löke S., Roelcke D., Hänsch G. M. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. 1986 Mar 1;136(5):1772–1776. [PubMed] [Google Scholar]
- Stefanová I., Hilgert I., Kristofová H., Brown R., Low M. G., Horejsí V. Characterization of a broadly expressed human leucocyte surface antigen MEM-43 anchored in membrane through phosphatidylinositol. Mol Immunol. 1989 Feb;26(2):153–161. doi: 10.1016/0161-5890(89)90097-7. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Nakano Y., Tomita M. Isolation from human erythrocytes of a new membrane protein which inhibits the formation of complement transmembrane channels. J Biochem. 1988 Oct;104(4):633–637. doi: 10.1093/oxfordjournals.jbchem.a122524. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Tobe T., Oda E., Tomita M., Yasukawa K., Yamaji N., Takemoto T., Furuichi K., Takayama M., Yano S. Molecular cloning and characterization of MACIF, an inhibitor of membrane channel formation of complement. J Biochem. 1989 Oct;106(4):555–557. doi: 10.1093/oxfordjournals.jbchem.a122893. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Wood L. M., Müller-Eberhard H. J. Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6975–6979. doi: 10.1073/pnas.83.18.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]