Abstract
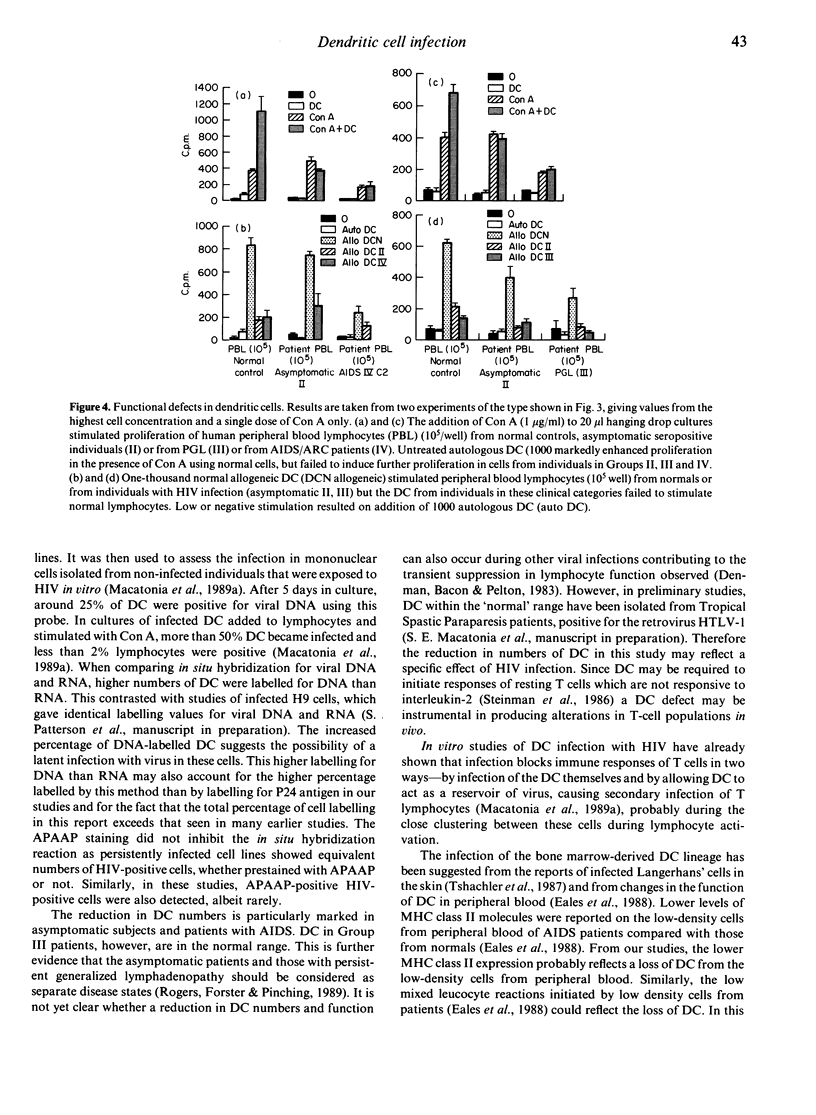
Immune responses in resting T cells are initiated by the presentation of antigen by bone marrow-derived dendritic cells (DC). Normal DC are susceptible to infection with human immunodeficiency virus (HIV) in vitro (Patterson & Knight, 1987) and this blocks their capacity to stimulate T-cell responses to other antigens (Macatonia, Patterson & Knight, 1989a). To study the relationship between HIV and DC in patients and its relevance to the pathogenesis of disease, DC have been isolated from the blood of individuals in the different clinical categories, counted, examined for the presence of virus genome and their antigen-presenting capacity measured. Infection, depletion and impaired function of DC occur in early HIV infection. HIV seropositive patients who were asymptomatic and those with symptoms of disease had significantly reduced numbers of DC, but patients with persistent generalized lymphadenopathy had normal numbers. Between 3% and 21% of DC, identified as large low-density cells not bearing monocyte, lymphocyte or natural killer cell markers, were infected with HIV, as indicated by in situ hybridization. Less than 0.12% of the lymphocytes or monocytes were infected. The DC from infected individuals were poor at enhancing responses to the mitogen concanavalin A (Con A). They also caused low levels of stimulation in allogeneic lymphocytes in mixed leucocyte cultures. By contrast, T cells from asymptomatic patients gave normal T-cell responses to uninfected allogeneic DC, although those from acquired immunodeficiency syndrome (AIDS) patients did show reduced responsiveness. Defects in DC thus precede both the appearance of symptoms and changes in T cells and may be instrumental in the development of AIDS. Furthermore, since DC numbers and function differ at different stages of disease, monitoring these may contribute to clinical assessment and lead to new therapeutic approaches.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Horne R. Follicular dendritic cells and virus-like particles in AIDS-related lymphadenopathy. Lancet. 1984 Aug 18;2(8399):370–372. doi: 10.1016/s0140-6736(84)90540-3. [DOI] [PubMed] [Google Scholar]
- Eales L. J., Farrant J., Helbert M., Pinching A. J. Peripheral blood dendritic cells in persons with AIDS and AIDS related complex: loss of high intensity class II antigen expression and function. Clin Exp Immunol. 1988 Mar;71(3):423–427. [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Arya S. K., Popovic M., Gallo R. C., Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984 Nov 8;312(5990):166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Pomerantz R. J., Kaplan J. C. Pathogenesis of infection with human immunodeficiency virus. N Engl J Med. 1987 Jul 30;317(5):278–286. doi: 10.1056/NEJM198707303170505. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Macatonia S. E. Dendritic cells and viruses. Immunol Lett. 1988 Nov;19(3):177–181. doi: 10.1016/0165-2478(88)90140-x. [DOI] [PubMed] [Google Scholar]
- Macatonia S. E., Patterson S., Knight S. C. Suppression of immune responses by dendritic cells infected with HIV. Immunology. 1989 Jul;67(3):285–289. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Taylor P. M., Knight S. C., Askonas B. A. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T cell responses in vitro. J Exp Med. 1989 Apr 1;169(4):1255–1264. doi: 10.1084/jem.169.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Gendelman H. E. Effects of colony stimulating factors on the interaction of monocytes and the human immunodeficiency virus. Immunol Lett. 1988 Nov;19(3):193–198. doi: 10.1016/0165-2478(88)90142-3. [DOI] [PubMed] [Google Scholar]
- Patterson S., Knight S. C. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J Gen Virol. 1987 Apr;68(Pt 4):1177–1181. doi: 10.1099/0022-1317-68-4-1177. [DOI] [PubMed] [Google Scholar]
- Ranki A., Valle S. L., Krohn M., Antonen J., Allain J. P., Leuther M., Franchini G., Krohn K. Long latency precedes overt seroconversion in sexually transmitted human-immunodeficiency-virus infection. Lancet. 1987 Sep 12;2(8559):589–593. doi: 10.1016/s0140-6736(87)92985-0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rogers L. A., Forster S. M., Pinching A. J. IgD production and other lymphocyte functions in HIV infection: immaturity and activation of B cells at different clinical stages. Clin Exp Immunol. 1989 Jan;75(1):7–11. [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Gigli I., Baer R. L., Thorbecke G. J. Langerhans cells: role in contact hypersensitivity and relationship to lymphoid dendritic cells and to macrophages. Immunol Rev. 1980;53:203–232. doi: 10.1111/j.1600-065x.1980.tb01045.x. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Inaba K. Stimulation of the primary mixed leukocyte reaction. Crit Rev Immunol. 1985;5(4):331–348. [PubMed] [Google Scholar]
- Tenner-Racz K., Racz P., Bofill M., Schulz-Meyer A., Dietrich M., Kern P., Weber J., Pinching A. J., Veronese-Dimarzo F., Popovic M. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986 Apr;123(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Tschachler E., Groh V., Popovic M., Mann D. L., Konrad K., Safai B., Eron L., diMarzo Veronese F., Wolff K., Stingl G. Epidermal Langerhans cells--a target for HTLV-III/LAV infection. J Invest Dermatol. 1987 Feb;88(2):233–237. doi: 10.1111/1523-1747.ep12525402. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. N., Wadsworth J., Rogers L. A., Moshtael O., Scott K., McManus T., Berrie E., Jeffries D. J., Harris J. R., Pinching A. J. Three-year prospective study of HTLV-III/LAV infection in homosexual men. Lancet. 1986 May 24;1(8491):1179–1182. doi: 10.1016/s0140-6736(86)91160-8. [DOI] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988 Jan;111(1):167–182. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]