Abstract
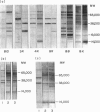
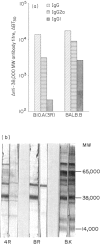
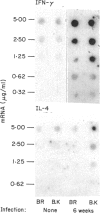
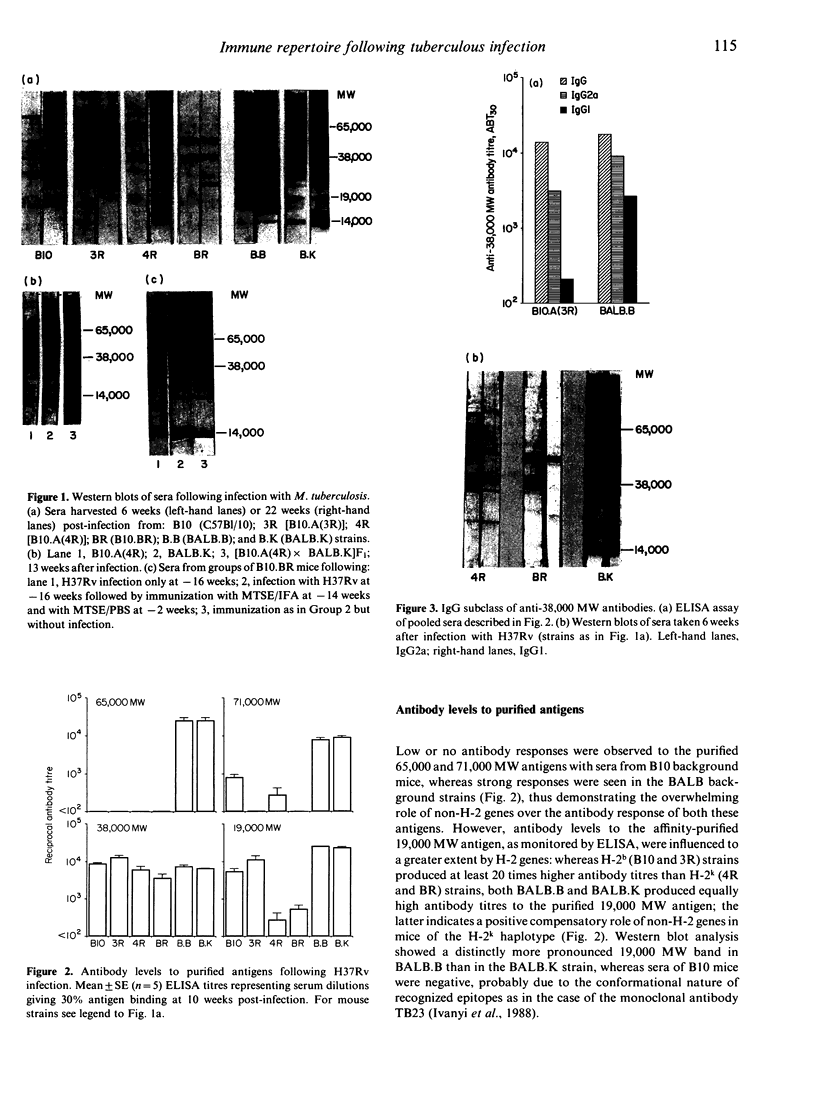
Differences in the antibody repertoire between B10 and BALB background strains have been found following intraperitoneal infection of mice with Mycobacterium tuberculosis. Western blot analysis showed that B10 sera reacted with only a few antigenic bands, whereas BALB sera reacted with multiple bands, irrespective of the H-2 (b or k) haplotype. The oligo-banded pattern was a feature of live infection, since immunization with the mycobacterial extract in incomplete Freunds' adjuvant (IFA) produced a multi-banded response in both intact and in previously infected B10 mice. The multibanded BALB phenotype was dominantly expressed in (BALB x B10) F1 hybrids. The extent to which antibody levels to individual antigens varied was influenced by the combined effects of background (non-H-2) and H-2 genes: anti-65,000 molecular weight (MW) and anti-71,000 MW IgG levels were high in BALB but absent or low in B10 mice, irrespective of H-2 haplotypes, anti-19,000 MW levels in B10 mice were strongly H-2 controlled, whilst anti-38,000 MW levels were high in all tested strains. Immunoglobulin G1 (IgG1) subclass antibody and splenic interleukin-4 (IL-4) mRNA levels were distinctly lower in B10 than in BALB mice, but in vitro T-cell proliferative responses to mycobacterial antigens did not differ between the tested strains. It is proposed that the limited extracellular release of mycobacterial antigens required to stimulate B cells and/or differential activation of T-cell subsets may explain the narrow antibody repertoire in B10 strains of mice. This may have relevance to the outcome of infection, as bacterial counts were higher in the BALB strains at 10 weeks post-infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Abou-Zeid C., Smith I., Grange J. M., Ratliff T. L., Steele J., Rook G. A. The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988 Feb;134(2):531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- Andersen A. B., Hansen E. B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989 Aug;57(8):2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbridge K. R., Booth R. J., Watson J. D., Lathigra R. B. Nucleotide sequence of the 19 kDa antigen gene from Mycobacterium tuberculosis. Nucleic Acids Res. 1989 Feb 11;17(3):1249–1249. doi: 10.1093/nar/17.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothamley G. H., Beck J. S., Schreuder G. M., D'Amaro J., de Vries R. R., Kardjito T., Ivanyi J. Association of tuberculosis and M. tuberculosis-specific antibody levels with HLA. J Infect Dis. 1989 Mar;159(3):549–555. doi: 10.1093/infdis/159.3.549. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Butler R. Interactions of Mycobacterium lepraemurium with resident peritoneal macrophages; phagocytosis and stimulation of the oxidative burst. Clin Exp Immunol. 1988 Jan;71(1):32–38. [PMC free article] [PubMed] [Google Scholar]
- Brett S. J., Butler R. Resistance to Mycobacterium lepraemurium is correlated with the capacity to generate macrophage activating factor(s) in response to mycobacterial antigens in vitro. Immunology. 1986 Nov;59(3):339–345. [PMC free article] [PubMed] [Google Scholar]
- Buschman E., Apt A. S., Nickonenko B. V., Moroz A. M., Averbakh M. H., Skamene E. Genetic aspects of innate resistance and acquired immunity to mycobacteria in inbred mice. Springer Semin Immunopathol. 1988;10(4):319–336. doi: 10.1007/BF02053844. [DOI] [PubMed] [Google Scholar]
- Chiplunkar S., De Libero G., Kaufmann S. H. Mycobacterium leprae-specific Lyt-2+ T lymphocytes with cytolytic activity. Infect Immun. 1986 Dec;54(3):793–797. doi: 10.1128/iai.54.3.793-797.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Curtis J., Turk J. L. Mitsuda-type lepromin reactions as a measure of host resistance in Mycobacterium lepraemurium infection. Infect Immun. 1979 May;24(2):492–500. doi: 10.1128/iai.24.2.492-500.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espitia C., Cervera I., González R., Mancilla R. A 38-kD Mycobacterium tuberculosis antigen associated with infection. Its isolation and serologic evaluation. Clin Exp Immunol. 1989 Sep;77(3):373–377. [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Palfliet K., Jurion F., Hilgers J., ten Berg R., Van Vooren J. P., De Bruyn J. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1988 Dec;56(12):3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986 Nov;59(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K., Jackett P., Bothamley G. Immunological study of the defined constituents of mycobacteria. Springer Semin Immunopathol. 1988;10(4):279–300. doi: 10.1007/BF02053841. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungqvist L., Worsaae A., Heron I. Antibody responses against Mycobacterium tuberculosis in 11 strains of inbred mice: novel monoclonal antibody specificities generated by fusions, using spleens from BALB.B10 and CBA/J mice. Infect Immun. 1988 Aug;56(8):1994–1998. doi: 10.1128/iai.56.8.1994-1998.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlert A., Young D. B. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71kD antigen, a member of the 70kD heat-shock protein family. Mol Microbiol. 1989 Feb;3(2):125–130. doi: 10.1111/j.1365-2958.1989.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Shigekane V. M., Fisher W. L., Locksley R. M. Cellular and humoral immunity to Leishmania major in genetically susceptible mice after in vivo depletion of L3T4+ T cells. J Immunol. 1987 Aug 15;139(4):1303–1309. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. P., Mehra N. K., Dingley H. B., Pande J. N., Vaidya M. C. Human leukocyte antigen (HLA)-linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J Infect Dis. 1983 Oct;148(4):676–681. doi: 10.1093/infdis/148.4.676. [DOI] [PubMed] [Google Scholar]
- Stern J. J., Oca M. J., Rubin B. Y., Anderson S. L., Murray H. W. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988 Jun 1;140(11):3971–3977. [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]