Abstract
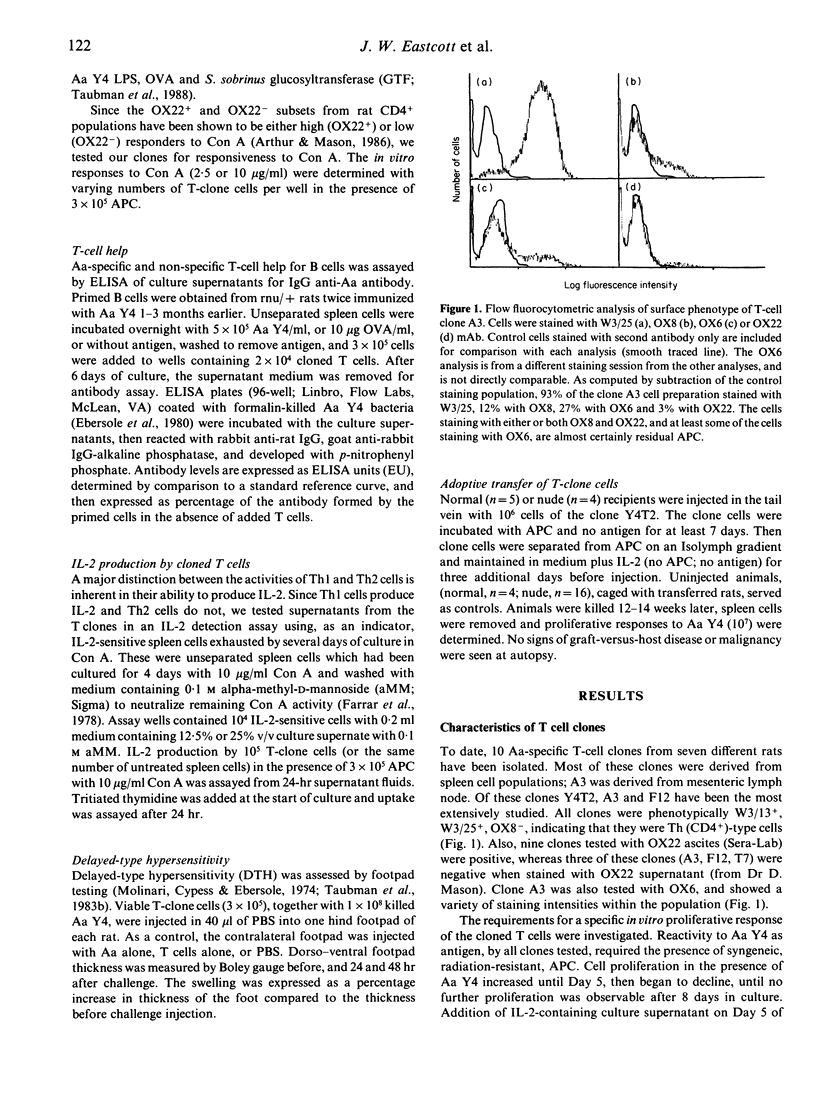
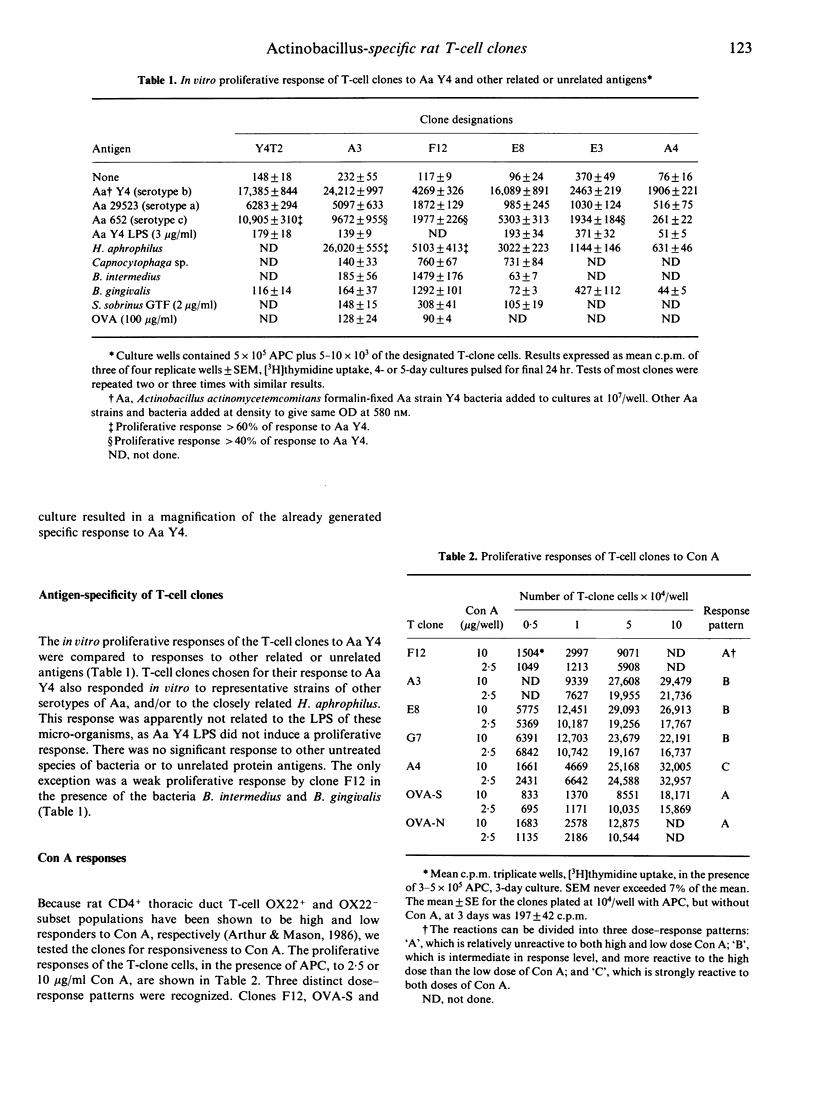
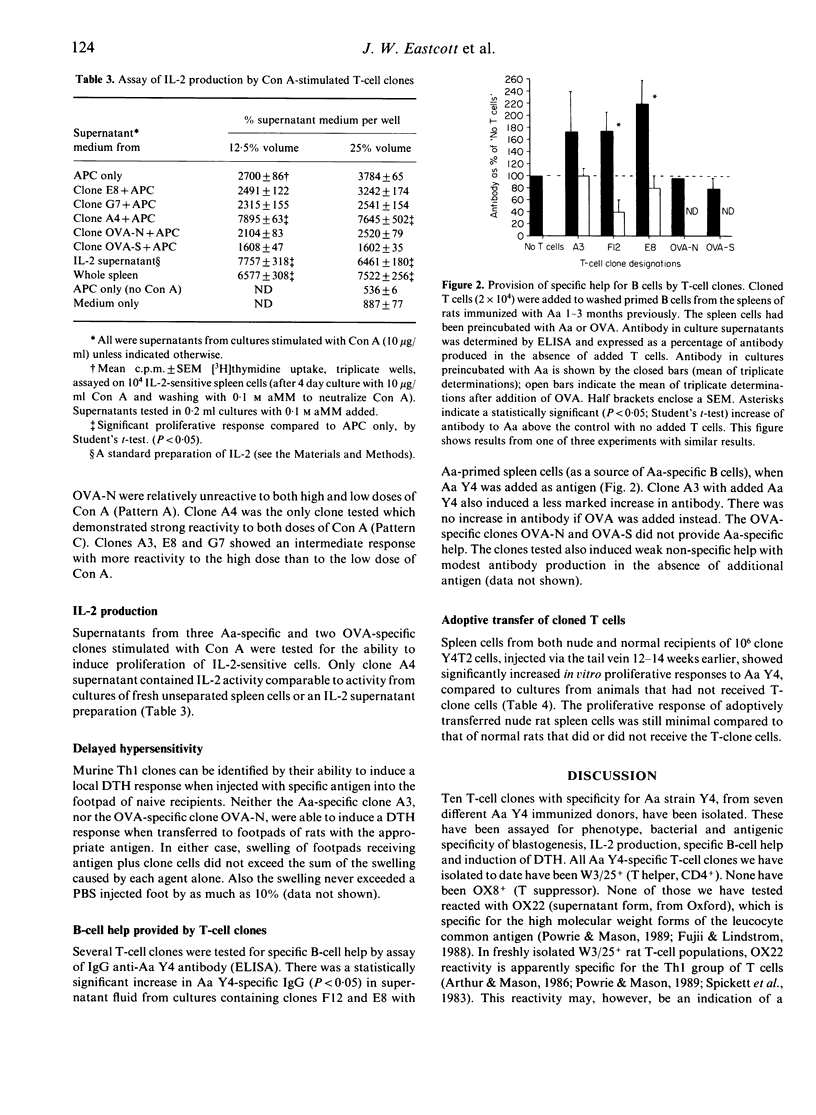
We have isolated 10 rat T-cell clones from the spleen or lymph nodes of seven different donors. These rats were immunized with 2-5 x 10(8) killed Actinobacillus actinomycetemcomitans (Aa) bacteria, injected either subcutaneously (s.c.) in complete Freund's adjuvant or intraperitoneally (i.p.) in saline. Clones studied to date have demonstrated a T-helper (Th) phenotype W3/13+, W3/25+, OX8- and OX22-. Clones were not stimulated in vitro by purified Aa-lipopolysaccharide (LPS) or heterologous Gram-negative bacteria, but proliferated when stimulated by bacteria representative of each of the three serological groups of Actinobacillus, indicating specificity for an Actinobacillus-common antigen other than LPS. One clone (A4) proliferated vigorously when stimulated with concanavalin A (Con A) in vitro, produced interleukin-2 (IL-2) and was provisionally classified as a Th1 type. This appears to be one of the few Th1-type rat clones reported. All other clones tested did not produce IL-2, exhibited B-cell help to some extent, did not induce delayed-type hypersensitivity (DTH) when injected into the footpads of naive rats along with the specific antigen, and were classified as Th2 type. Adoptive transfer of 10(6) cells of one Th2-type Aa-specific clone into syngeneic recipients resulted in a specific splenocyte in vitro response to Aa 12-14 weeks after cell transfer, indicating survival of cloned cells in recipient animals. The use of such clones in studies of experimental periodontal disease is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur R. P., Mason D. T cells that help B cell responses to soluble antigen are distinguishable from those producing interleukin 2 on mitogenic or allogeneic stimulation. J Exp Med. 1986 Apr 1;163(4):774–786. doi: 10.1084/jem.163.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Bottomly K., Luqman M., Greenbaum L., Carding S., West J., Pasqualini T., Murphy D. B. A monoclonal antibody to murine CD45R distinguishes CD4 T cell populations that produce different cytokines. Eur J Immunol. 1989 Apr;19(4):617–623. doi: 10.1002/eji.1830190407. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Chou Y. K., Vandenbark A. A., Jones R. E., Hashim G., Offner H. Selection of encephalitogenic rat T-lymphocyte clones recognizing an immunodominant epitope on myelin basic protein. J Neurosci Res. 1989 Feb;22(2):181–187. doi: 10.1002/jnr.490220211. [DOI] [PubMed] [Google Scholar]
- Eastcott J. W., Taubman M. A., Smith D. J., Holt S. C. Actinobacillus actinomycetemcomitans mitogenicity for B cells can be attributed to lipopolysaccharide. Oral Microbiol Immunol. 1990 Feb;5(1):8–11. doi: 10.1111/j.1399-302x.1990.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Frey D. E., Taubman M. A., Smith D. J. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J Periodontal Res. 1980 Nov;15(6):621–632. doi: 10.1111/j.1600-0765.1980.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Simon P. L., Koopman W. J., Fuller-Bonar J. Biochemical relationship of thymocyte mitogenic factor and factors enhancing humoral and cell-mediated immune responses. J Immunol. 1978 Oct;121(4):1353–1360. [PubMed] [Google Scholar]
- Fujii Y., Lindstrom J. Regulation of antibody production by helper T cell clones in experimental autoimmune myasthenia gravis. J Immunol. 1988 Nov 15;141(10):3361–3369. [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Antigen presentation by cells that are not of bone marrow origin. Reg Immunol. 1989 Jan-Feb;2(1):60–71. [PubMed] [Google Scholar]
- Hirose W., Davies T. F. Generation of thyroglobulin-specific T-cell clones derived from F1 Fisher rats. Immunology. 1988 May;64(1):107–112. [PMC free article] [PubMed] [Google Scholar]
- Katsuki M., Kakimoto K., Kawata S., Kotani S., Koga T. Induction of delayed-type hypersensitivity by the T cell line specific to bacterial peptidoglycans. J Immunol. 1987 Dec 1;139(11):3570–3572. [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kiley P., Holt S. C. Characterization of the lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4 and N27. Infect Immun. 1980 Dec;30(3):862–873. doi: 10.1128/iai.30.3.862-873.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto M., Fathman C. G. Antigen-reactive T cell clones. I. Transcomplementing hybrid I-A-region gene products function effectively in antigen presentation. J Exp Med. 1980 Oct 1;152(4):759–770. doi: 10.1084/jem.152.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman A. H., Chin J., Schmidt J. A., Abbas A. K. Role of interleukin 1 in the activation of T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9699–9703. doi: 10.1073/pnas.85.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari J. A., Cypess R. H., Ebersole J. L. Effect of Trichinella spiralis infection on the cell-mediated immune response to BCG. Int Arch Allergy Appl Immunol. 1974;47(4):483–487. doi: 10.1159/000231242. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Offner H., Hashim G. A., Chou Y. K., Celnik B., Jones R., Vandenbark A. A. Encephalitogenic T cell clones with variant receptor specificity. J Immunol. 1988 Dec 1;141(11):3828–3832. [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. The MRC OX-22- CD4+ T cells that help B cells in secondary immune responses derive from naive precursors with the MRC OX-22+ CD4+ phenotype. J Exp Med. 1989 Mar 1;169(3):653–662. doi: 10.1084/jem.169.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D., MacPhee I. A., Puklavec M. Isolation of encephalitogenic CD4+ T cell clones in the rat. Cloning methodology and interferon-gamma secretion. J Immunol Methods. 1989 Jul 26;121(2):185–196. doi: 10.1016/0022-1759(89)90159-2. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Ebersole J. L., Smith D. J. Immunoglobulins of rnu/rnu 'nude' rats with congenital aplasia of the thymus. Immunology. 1983 Jun;49(2):295–300. [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J., King W. F., Eastcott J. W., Bergey E. J., Levine M. J. Immune properties of glucosyltransferases from S. sobrinus. J Oral Pathol. 1988 Nov;17(9-10):466–470. doi: 10.1111/j.1600-0714.1988.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Taubman M. A., Yoshie H., Wetherell J. R., Jr, Ebersole J. L., Smith D. J. Immune response and periodontal bone loss in germfree rats immunized and infected with Actinobacillus actinomycetemcomitans. J Periodontal Res. 1983 Jul;18(4):393–401. doi: 10.1111/j.1600-0765.1983.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Yoshie H., Taubman M. A., Ebersole J. L., Smith D. J., Olson C. L. Periodontal bone loss and immune characteristics of congenitally athymic and thymus cell-reconstituted athymic rats. Infect Immun. 1985 Nov;50(2):403–408. doi: 10.1128/iai.50.2.403-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie H., Taubman M. A., Olson C. L., Ebersole J. L., Smith D. J. Periodontal bone loss and immune characteristics after adoptive transfer of Actinobacillus-sensitized T cells to rats. J Periodontal Res. 1987 Nov;22(6):499–505. doi: 10.1111/j.1600-0765.1987.tb02061.x. [DOI] [PubMed] [Google Scholar]
- Zambon J. J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985 Jan;12(1):1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Slots J., Genco R. J. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983 Jul;41(1):19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Umemoto T., De Nardin E., Nakazawa F., Christersson L. A., Genco R. J. Actinobacillus actinomycetemcomitans in the pathogenesis of human periodontal disease. Adv Dent Res. 1988 Nov;2(2):269–274. doi: 10.1177/08959374880020021101. [DOI] [PubMed] [Google Scholar]


