Abstract
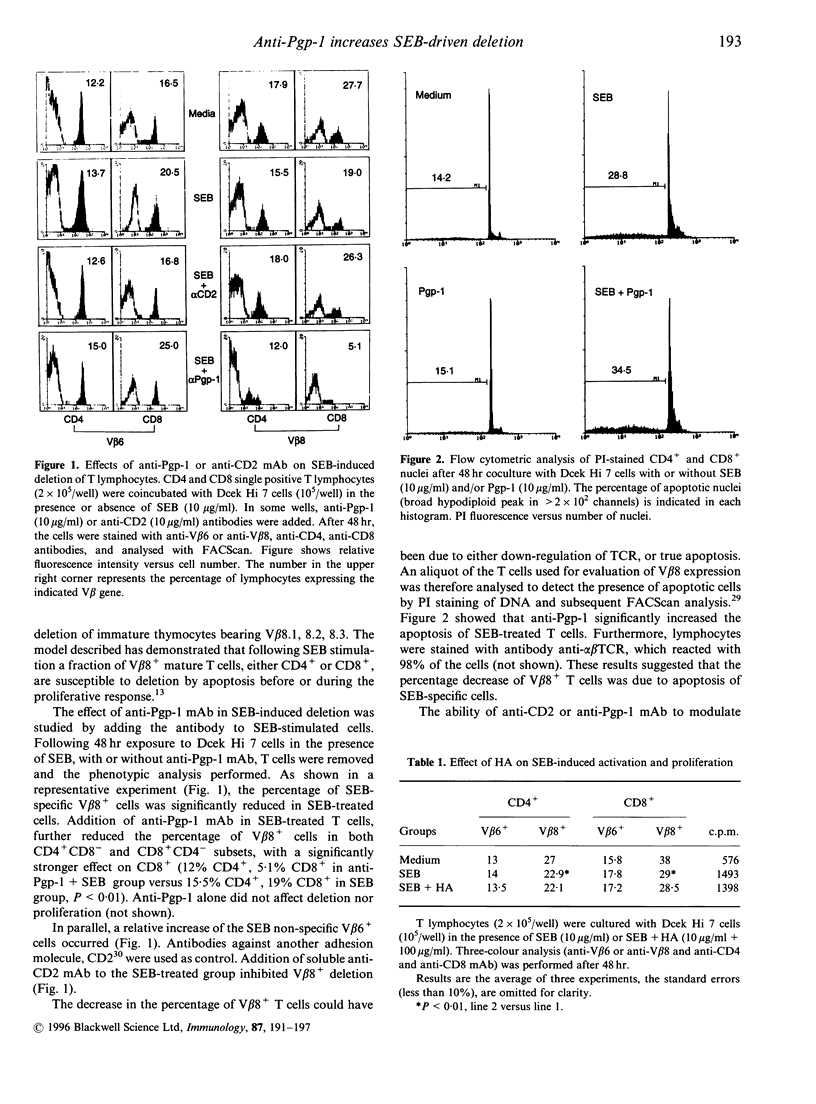
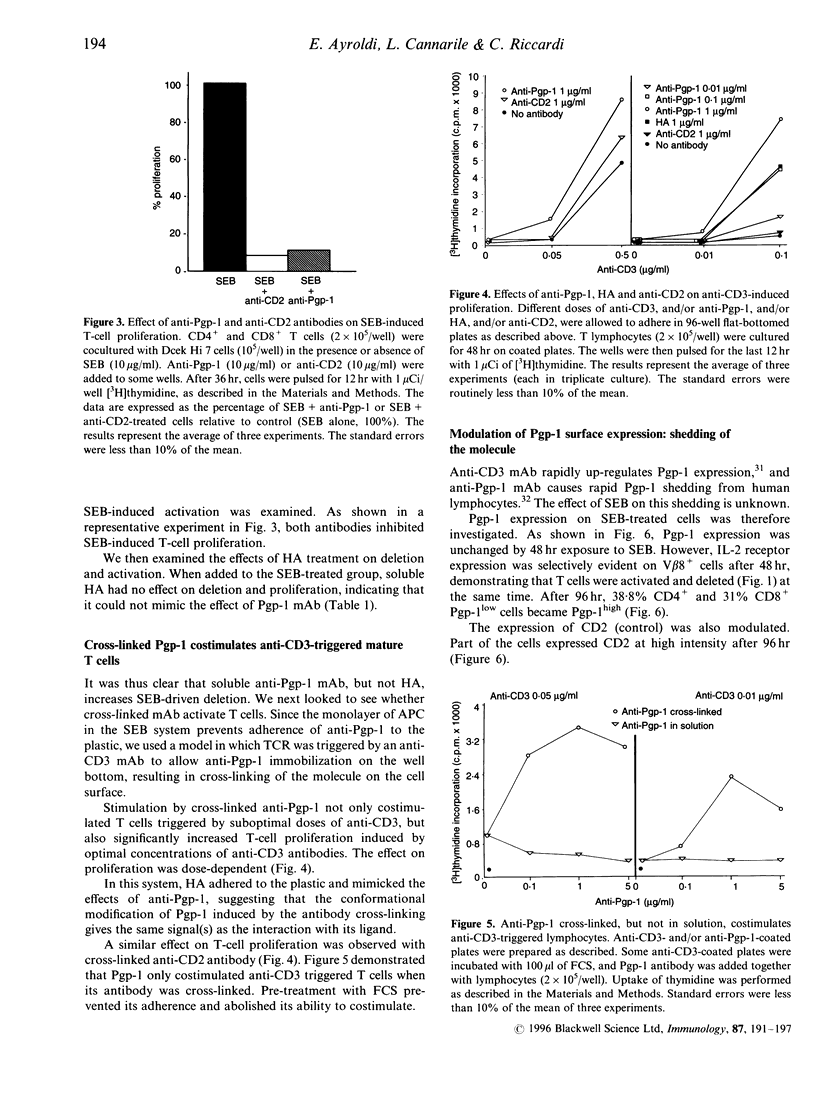
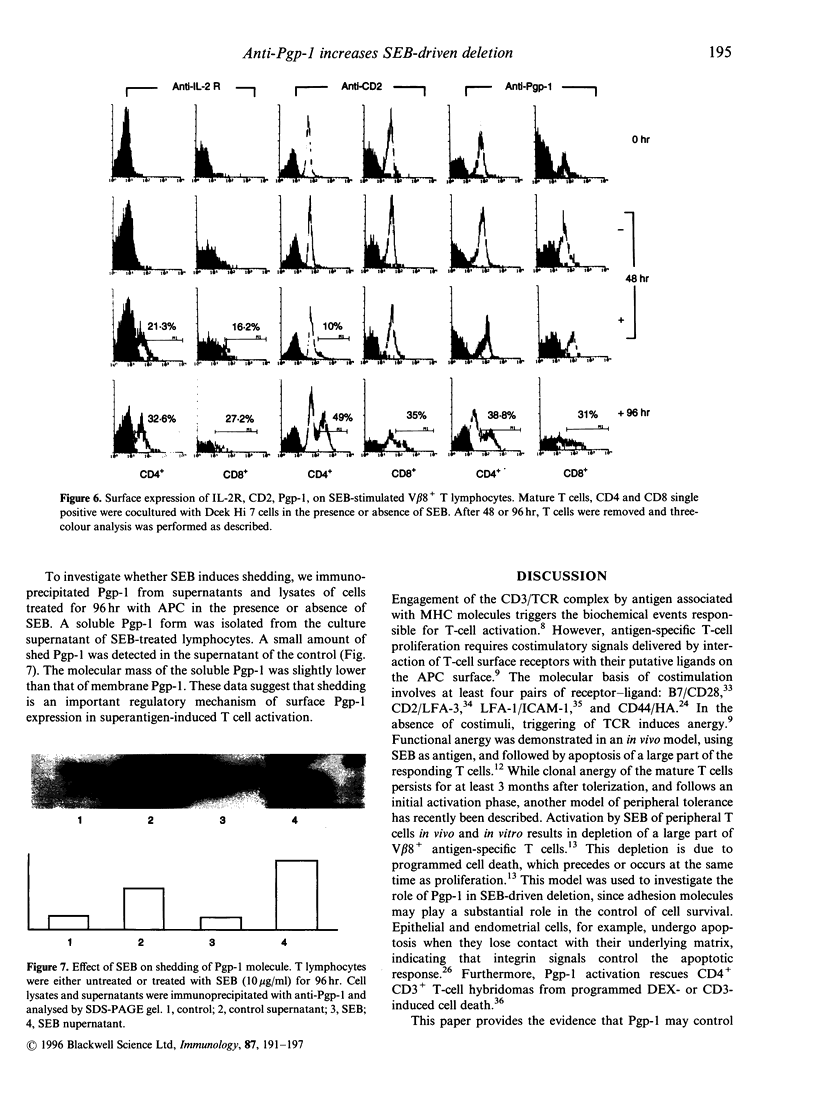
We examined the effects of anti-Pgp-1 (CD44) antibody on the in vitro deletion of murine CD4 and CD8 single positive T cells induced by Staphylococcal enterotoxin B (SEB). Soluble anti-Pgp-1 antibody enhanced the apoptosis and decreased the proliferation of SEB-responding T cells. In contrast, cross-linked anti-Pgp-1 antibody provided costimulatory signals for the T-cell activation induced by anti-CD3 antibody. Hyaluronic acid (HA), a ligand of Pgp-1, did not affect proliferation and deletion induced by SEB, whereas it mimicked the effects of the cross-linked antibody in anti-CD3-driven proliferation. T-cell Pgp-1 surface expression after 48 hr incubation with SEB was unchanged as compared to unstimulated cells. However, when the memory T cells were established, some V beta 8+ (SEB-specific) T cells Pgp-1low became Pgp-1high, displaying a bimodal character. Moreover, the Pgp-1 increased expression correlated with an increase of Pgp-1 soluble form in the supernatant. These findings suggested that signals following the triggering of the Pgp-1 molecule are important in controlling T-cell survival.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Ayroldi E., Cannarile L., D'Adamio F., Riccardi C. Superantigen-induced peripheral T-cell deletion: the effects of chemical modification of antigen-presenting cells, interleukin-4 and glucocorticoid hormones. Immunology. 1995 Apr;84(4):528–535. [PMC free article] [PubMed] [Google Scholar]
- Ayroldi E., Cannarile L., Migliorati G., Bartoli A., Nicoletti I., Riccardi C. CD44 (Pgp-1) inhibits CD3 and dexamethasone-induced apoptosis. Blood. 1995 Oct 1;86(7):2672–2678. [PubMed] [Google Scholar]
- Bazil V., Horejsí V. Shedding of the CD44 adhesion molecule from leukocytes induced by anti-CD44 monoclonal antibody simulating the effect of a natural receptor ligand. J Immunol. 1992 Aug 1;149(3):747–753. [PubMed] [Google Scholar]
- Carlow D. A., Teh S. J., van Oers N. S., Miller R. G., Teh H. S. Peripheral tolerance through clonal deletion of mature CD4-CD8+ T cells. Int Immunol. 1992 May;4(5):599–610. doi: 10.1093/intimm/4.5.599. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A. Characterization of the class III collagen receptor, a phosphorylated, transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem. 1988 Mar 25;263(9):4193–4201. [PubMed] [Google Scholar]
- Conlon K. C., Ochoa A. C., Kopp W. C., Ortaldo J. R., Urba W. J., Longo D. L., Young H. A. Enhanced lymphokine production and lymphokine receptor expression in multiple antibody-stimulated human CD4+ peripheral blood lymphocytes. J Immunol. 1992 Nov 15;149(10):3278–3289. [PubMed] [Google Scholar]
- D'Adamio L., Awad K. M., Reinherz E. L. Thymic and peripheral apoptosis of antigen-specific T cells might cooperate in establishing self tolerance. Eur J Immunol. 1993 Mar;23(3):747–753. doi: 10.1002/eji.1830230327. [DOI] [PubMed] [Google Scholar]
- Goodman J. W., Sercarz E. E. The complexity of structures involved in T-cell activation. Annu Rev Immunol. 1983;1:465–498. doi: 10.1146/annurev.iy.01.040183.002341. [DOI] [PubMed] [Google Scholar]
- Guo Y. J., Lin S. C., Wang J. H., Bigby M., Sy M. S. Palmitoylation of CD44 interferes with CD3-mediated signaling in human T lymphocytes. Int Immunol. 1994 Feb;6(2):213–221. doi: 10.1093/intimm/6.2.213. [DOI] [PubMed] [Google Scholar]
- Hengartner H., Odermatt B., Schneider R., Schreyer M., Wälle G., MacDonald H. R., Zinkernagel R. M. Deletion of self-reactive T cells before entry into the thymus medulla. Nature. 1988 Nov 24;336(6197):388–390. doi: 10.1038/336388a0. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Zöller M., Pals S. T., Ponta H. CD44 splice variants: metastases meet lymphocytes. Immunol Today. 1993 Aug;14(8):395–399. doi: 10.1016/0167-5699(93)90141-7. [DOI] [PubMed] [Google Scholar]
- Huet S., Groux H., Caillou B., Valentin H., Prieur A. M., Bernard A. CD44 contributes to T cell activation. J Immunol. 1989 Aug 1;143(3):798–801. [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. A., Chin L. T., Longo D. L., Kruisbeek A. M. Peripheral clonal elimination of functional T cells. Science. 1990 Dec 21;250(4988):1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kawabe Y., Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991 Jan 17;349(6306):245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Krakauer T. Cell adhesion molecules are co-receptors for staphylococcal enterotoxin B-induced T-cell activation and cytokine production. Immunol Lett. 1994 Feb;39(2):121–125. doi: 10.1016/0165-2478(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Lesley J., Kincade P. W., Hyman R. Antibody-induced activation of the hyaluronan receptor function of CD44 requires multivalent binding by antibody. Eur J Immunol. 1993 Aug;23(8):1902–1909. doi: 10.1002/eji.1830230826. [DOI] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti I., Migliorati G., Pagliacci M. C., Grignani F., Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991 Jun 3;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., Zinkernagel R. M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989 Nov 30;342(6249):559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Porteu F., Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990 Aug 1;172(2):599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Sharrow S. O., Singer A. Clonal deletion and clonal anergy in the thymus induced by cellular elements with different radiation sensitivities. J Exp Med. 1990 Mar 1;171(3):935–940. doi: 10.1084/jem.171.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchese F., Schwartz R. H., Germain R. N. Functionally distinct subsites on a class II major histocompatibility complex molecule. Nature. 1987 Sep 17;329(6136):254–256. doi: 10.1038/329254a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Reed J. C. Anchorage dependence, integrins, and apoptosis. Cell. 1994 May 20;77(4):477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Guo Y. J., Stamenkovic I. Distinct effects of two CD44 isoforms on tumor growth in vivo. J Exp Med. 1991 Oct 1;174(4):859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Schulte R., Hyman R., Trotter J. Biochemical characterization and cellular distribution of a polymorphic, murine cell-surface glycoprotein expressed on lymphoid tissues. Immunogenetics. 1982 Mar;15(3):299–312. doi: 10.1007/BF00364338. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Willerford D. M., Hoffman P. A., Gallatin W. M. Expression of lymphocyte adhesion receptors for high endothelium in primates. Anatomic partitioning and linkage to activation. J Immunol. 1989 May 15;142(10):3416–3422. [PubMed] [Google Scholar]



