Abstract
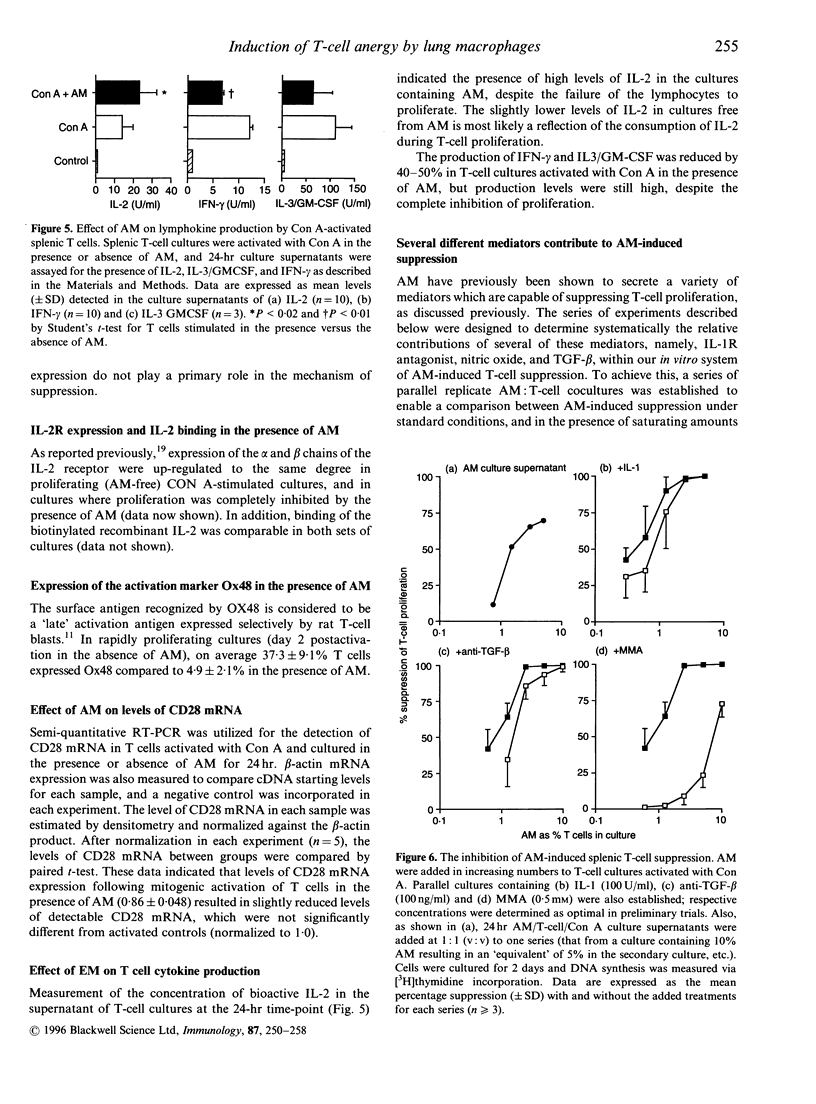
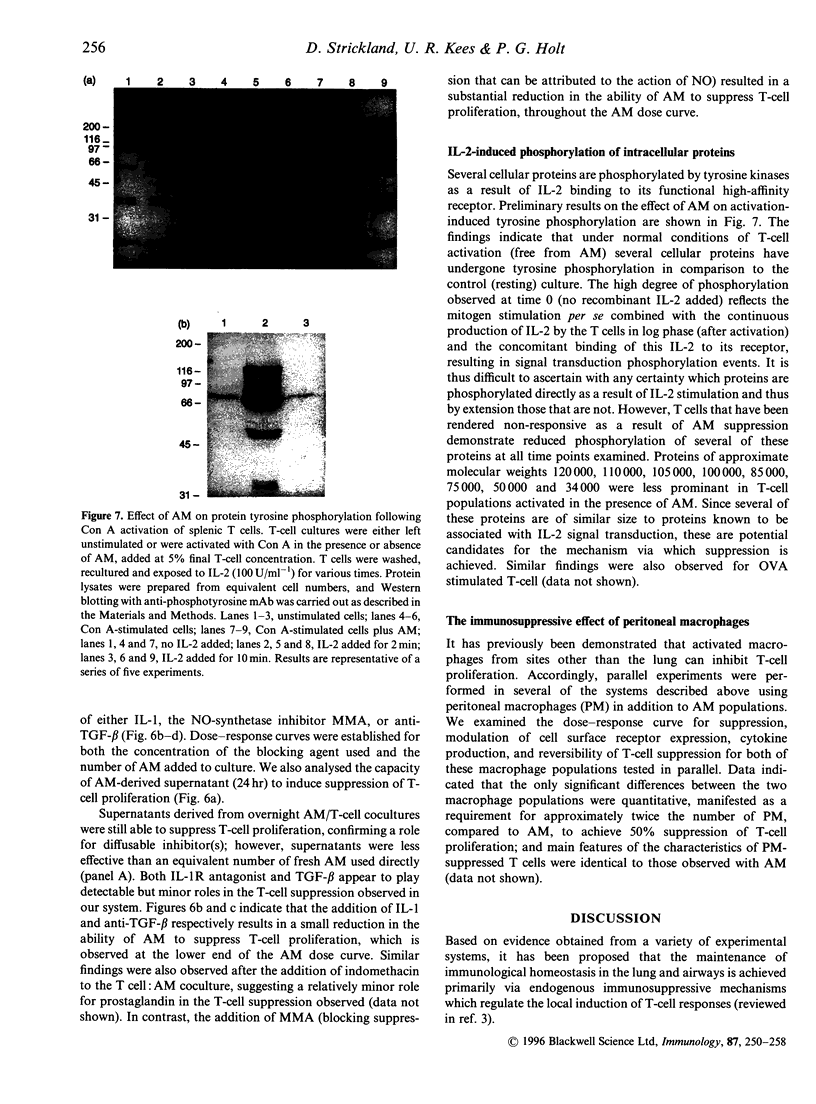
Alveolar macrophages (AM) are recognized as archetypal 'activated' macrophages with respect to their capacity to suppress T-cell responses to antigen or mitogen, and this function has been ascribed an important role in the maintenance of local immunological homeostasis at the delicate blood:air interface. The present study demonstrates that this suppression involves a unique form of T-cell anergy, in which 'AM-suppressed' T cells proceed normally through virtually all phases of the activation sequence including Ca2+ flux, T-cell receptor (TCR) modulation, cytokine [including interleukin-2 (IL-2)] secretion and IL-2 receptor (IL-2R) expression. However, the 'suppressed' T cells fail to up-regulate CD2, and do not re-express normal levels of TCR-associated molecules after initial down-modulation; moreover, they are unable to transduce IL-2 signals leading to phosphorylation of IL-2R-associated proteins, and remained locked in G0/G1. The induction of this form of anergy is blocked by an NO-synthase inhibitor, and is reversible upon removal of AM from the T cells, which then proliferate in the absence of further stimulation. We hypothesize that this mechanism provides the means to limit the magnitude of local immune responses in this fragile tissue microenvironment, while preserving the capacity for generation of immunological memory against locally encountered antigens via clonal expansion of activated T cells after their subsequent migration to regional lymphoid organs. In an accompanying paper, we demonstrate that a significant proportion of T cells freshly isolated from lung exhibit a comparable surface phenotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boussiotis V. A., Barber D. L., Nakarai T., Freeman G. J., Gribben J. G., Bernstein G. M., D'Andrea A. D., Ritz J., Nadler L. M. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science. 1994 Nov 11;266(5187):1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Verbi W., Davies A., Parker P., Crumpton M. J. Evidence that protein kinase C differentially regulates the human T lymphocyte CD2 and CD3 surface antigens. Eur J Immunol. 1988 Sep;18(9):1391–1396. doi: 10.1002/eji.1830180914. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Corradin G. High antigen concentration inhibits T cell proliferation but not interleukin 2 production: examination of limiting dilution microcultures and T cell clones. Eur J Immunol. 1986 Jan;16(1):30–34. doi: 10.1002/eji.1830160107. [DOI] [PubMed] [Google Scholar]
- Clark G. J., Dallman M. J. Identification of a cDNA encoding the rat CD28 homologue. Immunogenetics. 1992;35(1):54–57. doi: 10.1007/BF00216628. [DOI] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassel H. J., Hutchinson I. V., Tellides G., Knoop M., Hackmann J., Engemann R., Morris P. J. Phenotypic characterization of T-suppressor lymphocytes induced by orthotopic rat liver transplantation. Transplant Proc. 1989 Feb;21(1 Pt 1):429–430. [PubMed] [Google Scholar]
- Holt P. G. Regulation of antigen-presenting cell function(s) in lung and airway tissues. Eur Respir J. 1993 Jan;6(1):120–129. [PubMed] [Google Scholar]
- Holt P. G., Schon-Hegrad M. A., Oliver J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J Exp Med. 1988 Feb 1;167(2):262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot A. E., Moore A. L., Roberts J. D., Hacker M. P. Nitric oxide modulates lymphocyte proliferation but not secretion of IL-2. Immunol Invest. 1993 Jun;22(4):319–327. doi: 10.3109/08820139309063411. [DOI] [PubMed] [Google Scholar]
- Hünig T., Wallny H. J., Hartley J. K., Lawetzky A., Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989 Jan 1;169(1):73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A. Frequency analysis of lymphokine-secreting CD4+ and CD8+ T cells activated in a graft-versus-host reaction. J Immunol. 1990 Oct 1;145(7):2167–2176. [PubMed] [Google Scholar]
- Lake R. A., O'Hehir R. E., Verhoef A., Lamb J. R. CD28 mRNA rapidly decays when activated T cells are functionally anergized with specific peptide. Int Immunol. 1993 May;5(5):461–466. doi: 10.1093/intimm/5.5.461. [DOI] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Nau G. J., Moldwin R. L., Lancki D. W., Kim D. K., Fitch F. W. Inhibition of IL 2-driven proliferation of murine T lymphocyte clones by supraoptimal levels of immobilized anti-T cell receptor monoclonal antibody. J Immunol. 1987 Jul 1;139(1):114–122. [PubMed] [Google Scholar]
- Nicolls M. R., Aversa G. G., Pearce N. W., Spinelli A., Berger M. F., Gurley K. E., Hall B. M. Induction of long-term specific tolerance to allografts in rats by therapy with an anti-CD3-like monoclonal antibody. Transplantation. 1993 Mar;55(3):459–468. doi: 10.1097/00007890-199303000-00001. [DOI] [PubMed] [Google Scholar]
- O'Hehir R. E., Yssel H., Verma S., de Vries J. E., Spits H., Lamb J. R. Clonal analysis of differential lymphokine production in peptide and superantigen induced T cell anergy. Int Immunol. 1991 Aug;3(8):819–826. doi: 10.1093/intimm/3.8.819. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993 Apr 9;73(1):5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- Thepen T., McMenamin C., Oliver J., Kraal G., Holt P. G. Regulation of immune response to inhaled antigen by alveolar macrophages: differential effects of in vivo alveolar macrophage elimination on the induction of tolerance vs. immunity. Eur J Immunol. 1991 Nov;21(11):2845–2850. doi: 10.1002/eji.1830211128. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Upham J. W., Strickland D. H., Bilyk N., Robinson B. W., Holt P. G. Alveolar macrophages from humans and rodents selectively inhibit T-cell proliferation but permit T-cell activation and cytokine secretion. Immunology. 1995 Jan;84(1):142–147. [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Clark S. J., Paterson D. J., Willis A. C. Similarities in sequences and cellular expression between rat CD2 and CD4 antigens. J Exp Med. 1987 Feb 1;165(2):368–380. doi: 10.1084/jem.165.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meide P. H., Borman T. H., de Labie M. C., Wubben J. A., Botman C. A., Vijverberg K., Schellekens H. A sensitive two-site enzyme immunoassay for the detection of rat interferon-gamma in biological fluids. J Interferon Res. 1990 Apr;10(2):183–189. doi: 10.1089/jir.1990.10.183. [DOI] [PubMed] [Google Scholar]