Abstract
Thoracic duct lymphocytes (TDL) collected 3 days after infection of rats with Trichinella spiralis (TS) and adoptively transferred into normal, uninfected recipients, increased the numbers of both mucosal mast cells (MMC) and eosinophils (EOS) in the intestine. The CD4+ T-helper cell population was separated into two subsets (OX22+ and OX22-) using OX22 monoclonal antibody (mAb) and panning techniques. After adoptive transfer of these T-helper subsets i.v., rats were challenged with TS 24 hr later. The intestine of recipient rats was examined histologically at intervals from Day 3 to Day 21. On Day 9 after transfer, OX22+ T helpers induced a substantial mastocytosis [94 +/- 3, mean +/- SE/villus crypt unit (VCU)], whereas the OX22- T-helper subset increased resident EOS numbers (60 +/- 2/VCU) compared to the challenge control (18 +/- 1 MMC, 27 +/- 1 EOS/VCU). The time of peak eosinophilia was advanced by 3-6 days for recipients of OX22- cells and that of mast cells by 9-12 days for recipients of OX22+ cells. The recipients of OX22-, but not OX22+, cells also showed a large increase in the numbers of B cells in the spleen and mesenteric lymph node (MLN) secreting antibody against adult TS. Recipients of OX22- cells displayed an even increase in EOS throughout the villi, lamina propria (LP) and muscularis, whereas in OX22+ cell recipients mast cells were only present in the lower villus and the epithelium just above the crypt as well as the muscularis layer. Only the CD4+ OX22- cell subset conferred protection against TS in the intestine. We conclude that the OX22+ and OX22- T-helper cells exert distinctive effects in the intestine on MMC and EOS. Because protection was established in the presence of an OX22- T-helper-induced eosinophilia but without a concurrent mastocytosis, the results suggest that MMC are probably not involved in expulsion of TS to terminate the primary infection.
Full text
PDF


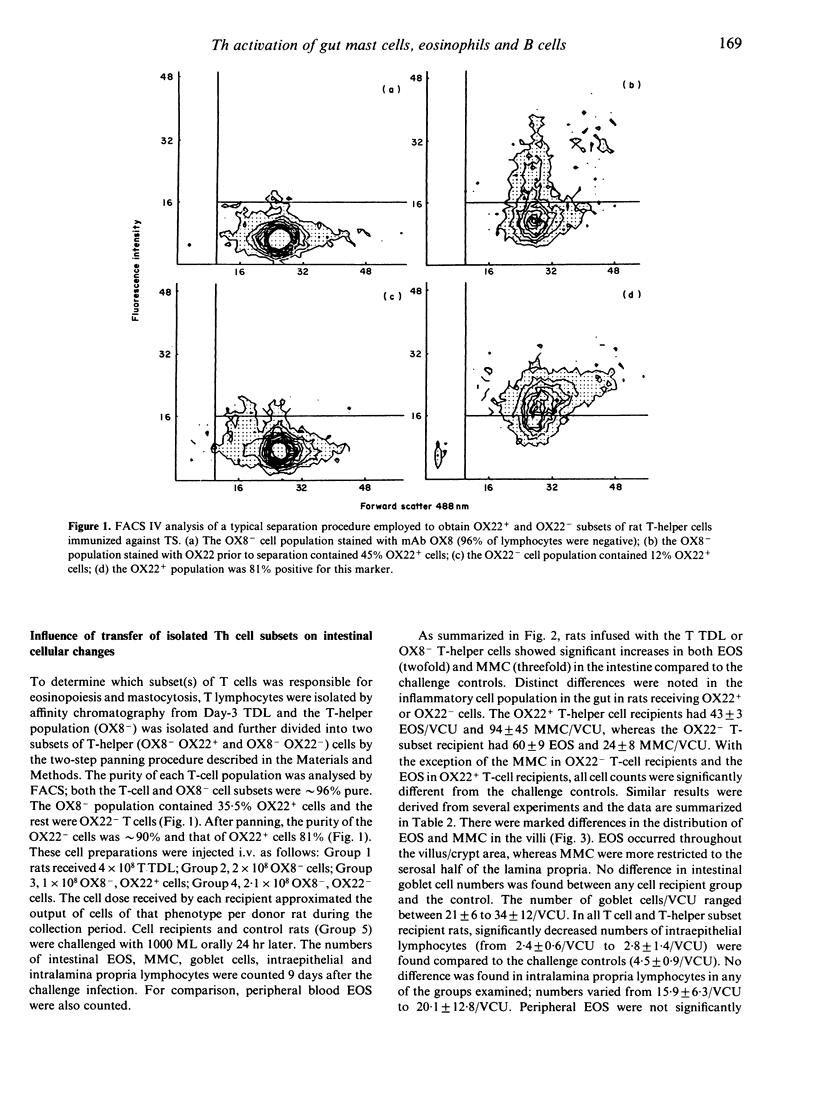
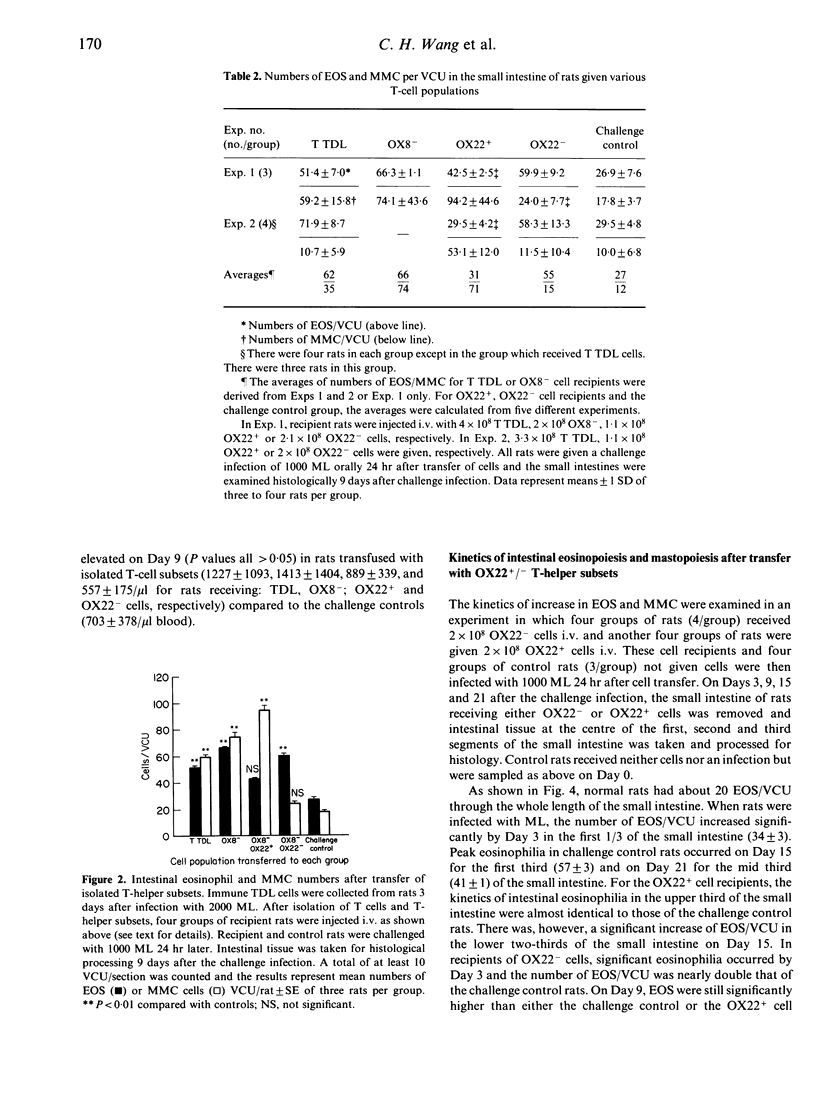
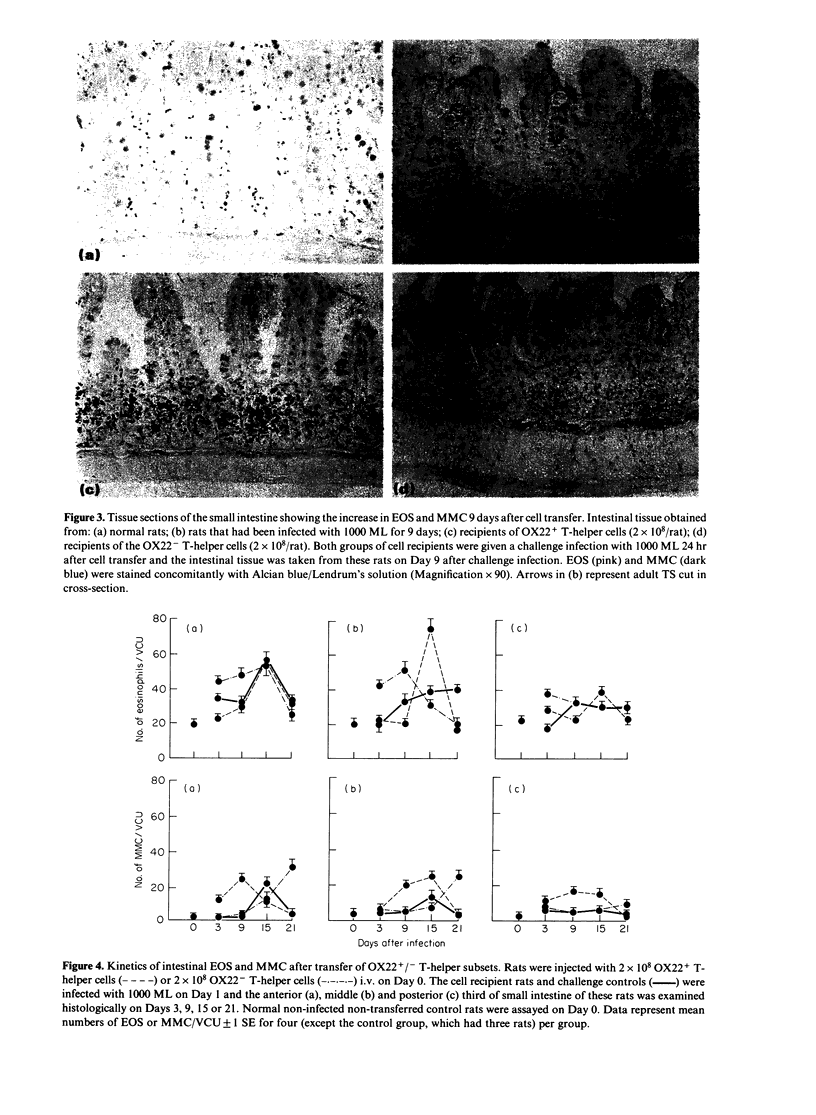
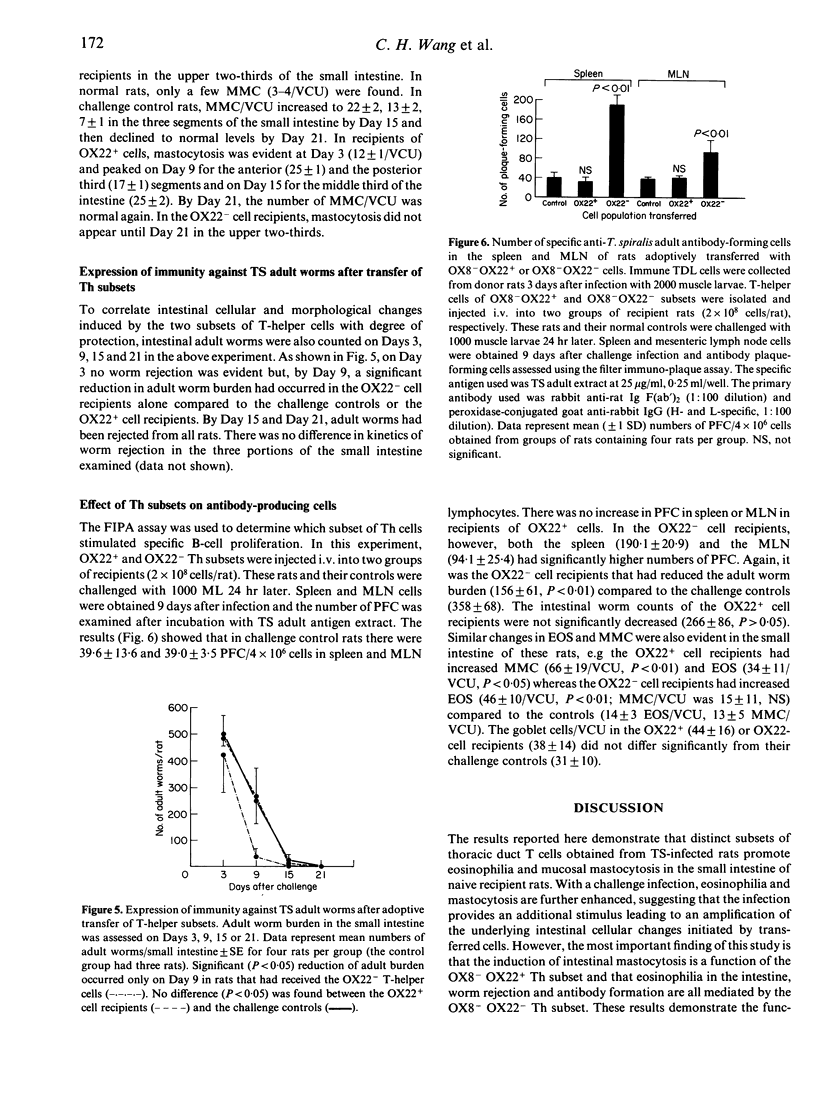



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlstedt S., Enander I. Immune regulation of goblet cell development. Int Arch Allergy Appl Immunol. 1987;82(3-4):357–360. doi: 10.1159/000234226. [DOI] [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Alizadeh H., Murrell K. D. The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient w/wv mice. J Parasitol. 1984 Oct;70(5):767–773. [PubMed] [Google Scholar]
- Appleton J. A., Schain L. R., McGregor D. D. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988 Nov;65(3):487–492. [PMC free article] [PubMed] [Google Scholar]
- Bell R. G., Korenaga M., Wang C. H. Characterization of a cell population in thoracic duct lymph that adoptively transfers rejection of adult Trichinella spiralis to normal rats. Immunology. 1987 Jun;61(2):221–227. [PMC free article] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D., Adams L. S. Trichinella spiralis: characterization and strain distribution of rapid expulsion in inbred mice. Exp Parasitol. 1982 Jun;53(3):301–314. doi: 10.1016/0014-4894(82)90073-x. [DOI] [PubMed] [Google Scholar]
- Bell R. G., McGregor D. D. Requirement for two discrete stimuli for induction of the intestinal rapid expulsion response against Trichinella spiralis in rats. Infect Immun. 1980 Jul;29(1):186–193. doi: 10.1128/iai.29.1.186-193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Clement L. T., Yamashita N., Martin A. M. The functionally distinct subpopulations of human CD4+ helper/inducer T lymphocytes defined by anti-CD45R antibodies derive sequentially from a differentiation pathway that is regulated by activation-dependent post-thymic differentiation. J Immunol. 1988 Sep 1;141(5):1464–1470. [PubMed] [Google Scholar]
- Crum E. D., Despommier D. D., McGregor D. D. Immunity to Trichinella spiralis. I. Transfer of resistance by two classes of lymphocytes. Immunology. 1977 Dec;33(6):787–795. [PMC free article] [PubMed] [Google Scholar]
- Crum E. D., McGregor D. D. Functional properties of T and B cells isolated by affinity chromatography from rat thoracic duct lymph. Cell Immunol. 1976 May;23(2):211–222. doi: 10.1016/0008-8749(76)90187-8. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Despommier D., Weisbroth S., Fass C. Circulating eosinophils and trichinosis in the rat: the parasitic stage responsible for induction during infection. J Parasitol. 1974 Apr;60(2):280–284. [PubMed] [Google Scholar]
- Dessein A. J., Parker W. L., James S. L., David J. R. IgE antibody and resistance to infection. I. Selective suppression of the IgE antibody response in rats diminishes the resistance and the eosinophil response to Trichinella spiralis infection. J Exp Med. 1981 Feb 1;153(2):423–436. doi: 10.1084/jem.153.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S. B., MacDonald T. T. Limit dilution analysis of mast cell precursor frequency in the gut epithelium of normal and Trichinella spiralis infected mice. Parasite Immunol. 1986 Sep;8(5):503–511. doi: 10.1111/j.1365-3024.1986.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Dy M., Luffau G., Vassalli P. Gut mucosal mast cells. Origin, traffic, and differentiation. J Exp Med. 1984 Jul 1;160(1):12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Korenaga M., Wang C. H., Bell R. G., Zhu D., Ahmad A. Intestinal immunity to Trichinella spiralis is a property of OX8- OX22- T-helper cells that are generated in the intestine. Immunology. 1989 Apr;66(4):588–594. [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Fisher R. Mast cells in severely T-cell depleted rats and the response to infestation with Nippostrongylus brasiliensis. Immunology. 1979 May;37(1):145–155. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Nawa Y. Nippostrongylus brasiliensis: intestinal goblet-cell response in adoptively immunized rats. Exp Parasitol. 1979 Feb;47(1):81–90. doi: 10.1016/0014-4894(79)90010-9. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Newlands G. F., Huntley J. F., Miller H. R. Concomitant detection of mucosal mast cells and eosinophils in the intestines of normal and Nippostrongylus-immune rats. A re-evaluation of histochemical and immunocytochemical techniques. Histochemistry. 1984;81(6):585–589. doi: 10.1007/BF00489539. [DOI] [PubMed] [Google Scholar]
- Parmentier H. K., de Vries C., Ruitenberg E. J., Van Loveren H. Involvement of serotonin in intestinal mastocytopoiesis and inflammation during a Trichinella spiralis infection in mice. Int Arch Allergy Appl Immunol. 1987;83(1):31–38. doi: 10.1159/000234327. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. The MRC OX-22- CD4+ T cells that help B cells in secondary immune responses derive from naive precursors with the MRC OX-22+ CD4+ phenotype. J Exp Med. 1989 Mar 1;169(3):653–662. doi: 10.1084/jem.169.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Morimoto C., Fitzgerald K. A., Hussey R. E., Daley J. F., Schlossman S. F. Heterogeneity of human T4+ inducer T cells defined by a monoclonal antibody that delineates two functional subpopulations. J Immunol. 1982 Jan;128(1):463–468. [PubMed] [Google Scholar]
- Rothwell T. L. Studies of the responses of basophil and eosinophil leucocytes and mast cells to the nematode Trichostrongylus colubriformis. I. Observations during the expulsion of first and second infections by guinea-pigs. J Pathol. 1975 May;116(1):51–60. doi: 10.1002/path.1711160109. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A., Kruizinga W., Leenstra F. Trichinella spiralis infection in congenitally athymic (nude) mice. Parasitological, serological and haematological studies with observations on intestinal pathology. Immunology. 1977 Oct;33(4):581–587. [PMC free article] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Dutoit E., Vernes A., Biguet J. Oral immunization of mice with metabolic antigens of Trichinella spiralis larvae: effects on the kinetics of intestinal cell response including mast cells and polymorphonuclear eosinophils. J Parasitol. 1979 Oct;65(5):685–691. [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of eosinophilia. Mouse strain variation in response to antigens of parasite origin. Clin Exp Immunol. 1983 Feb;51(2):239–246. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Wilson M. M. T and B cells in the transfer of immunity against Trichinella spiralis in mice. Immunology. 1979 May;37(1):103–109. [PMC free article] [PubMed] [Google Scholar]
- Wassom D. L., Johnson B. E., Sayles P. C. A filter immunoplaque assay for quantification of parasite-specific antibody producing cells. Parasitol Today. 1986 Aug;2(8):225–227. doi: 10.1016/0169-4758(86)90088-8. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R., Huntley J. F., Newlands G. F., Palliser A. C., Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. 1984 Nov 29-Dec 5Nature. 312(5993):450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- Woods A., West J., Rasmussen R., Bottomly K. Granulocyte-macrophage colony stimulating factor produced by cloned L3T4a+, class II-restricted T cells induces HT-2 cells to proliferate. J Immunol. 1987 Jun 15;138(12):4293–4297. [PubMed] [Google Scholar]



