Abstract
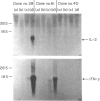
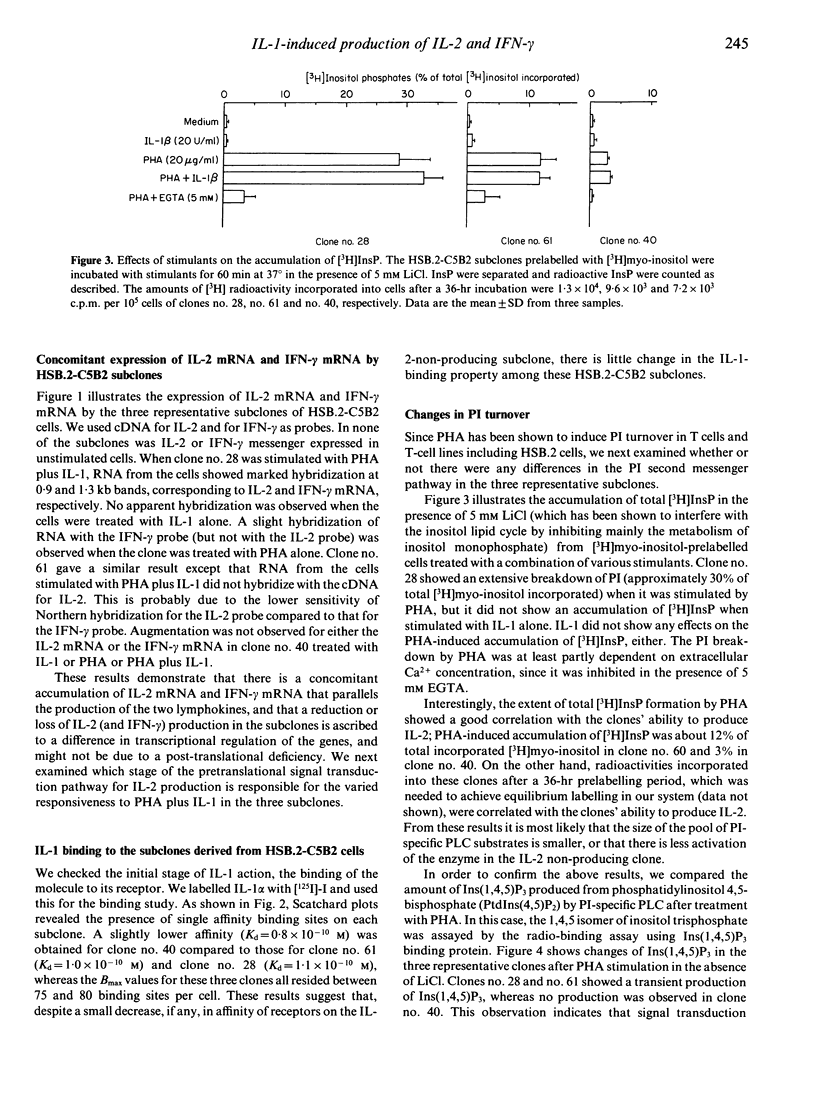
A human T-leukaemic cell line, HSB.2-C5B2, which produces high levels of interleukin-2 (IL-2) and interferon-gamma (IFN-gamma) when stimulated with phytohaemagglutinin (PHA) plus IL-1, was recloned to obtain spontaneous variants in IL-2 production in response to the stimuli. In these subclones, the ability of one clone to produce IL-2 correlated well with that to produce IFN-gamma. Three C5B2 subclones: clone no. 28, a high IL-2 producer, clone no. 61, an intermediate IL-2 producer, and clone no. 40, a non-producer, were selected and examined for differences in signal transduction mechanisms. Since the three subclones were shown to express about the same number of IL-1 binding sites with similar affinities, the loss of ability to produce IL-2 was not due to decreased cell-surface receptor or changes in receptor property. In support of this, IL-1 induced expression of the IL-2 receptor (Tac/p55 antigen) to the same extent on the three subclones. The levels of conventional intracellular second messengers were compared and it was revealed that loss of responsiveness was closely related to the subclones' degree of (poly)phosphoinositide (PI) turnover, protein kinase C (PKC) activation and cyclic AMP formation in response to PHA. Moreover, resting intracellular cyclic AMP concentrations were found to be increased in subclones with attenuated IL-2 production. These results indicate that the variation of IL-1-induced production of IL-2 and IFN-gamma in this T-cell line is attributed to the difference in the PHA-mediated signal transduction pathway and, presumably, to the different regulation of intracellular cyclic AMP.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aussel C., Mary D., Peyron J. F., Pelassy C., Ferrua B., Fehlmann M. Inhibition and activation of interleukin 2 synthesis by direct modification of guanosine triphosphate-binding proteins. J Immunol. 1988 Jan 1;140(1):215–220. [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Didier M., Aussel C., Pelassy C., Fehlmann M. IL-1 signaling for IL-2 production in T cells involves a rise in phosphatidylserine synthesis. J Immunol. 1988 Nov 1;141(9):3078–3080. [PubMed] [Google Scholar]
- Goldsmith M. A., Weiss A. Early signal transduction by the antigen receptor without commitment to T cell activation. Science. 1988 May 20;240(4855):1029–1031. doi: 10.1126/science.3259335. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Mukaida N., Hatake K., Motoyoshi K., Kawai T., Shiori-Nakano K. Interleukin 1 (IL 1)-dependent lymphokine production by human leukemic T cell line HSB.2 subclones. J Immunol. 1985 Mar;134(3):1682–1689. [PubMed] [Google Scholar]
- Kasahara T., Yagisawa H., Mukaida N., Matsumura H., Imai M., Shioiri-Nakano K. Interleukin 1 regulation of interleukin 2 and interferon-gamma gene activation in a human leukemic HSB.2 subclone. Microbiol Immunol. 1989;33(3):229–243. doi: 10.1111/j.1348-0421.1989.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kester M., Simonson M. S., Mené P., Sedor J. R. Interleukin-1 generates transmembrane signals from phospholipids through novel pathways in cultured rat mesangial cells. J Clin Invest. 1989 Feb;83(2):718–723. doi: 10.1172/JCI113937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Mary D., Aussel C., Ferrua B., Fehlmann M. Regulation of interleukin 2 synthesis by cAMP in human T cells. J Immunol. 1987 Aug 15;139(4):1179–1184. [PubMed] [Google Scholar]
- Matsushima K., Yodoi J., Tagaya Y., Oppenheim J. J. Down-regulation of interleukin 1 (IL 1) receptor expression by IL 1 and fate of internalized 125I-labeled IL 1 beta in a human large granular lymphocyte cell line. J Immunol. 1986 Nov 15;137(10):3183–3188. [PubMed] [Google Scholar]
- Mukaida N., Kasahara T., Hosoi J., Shioiri-Nakano K., Kawai T. Effects of anti-Tac antibody on the response of large granular lymphocytes to interleukin-2. Immunology. 1986 Jan;57(1):137–143. [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Kasahara T., Yagisawa H., Shioiri-Nakano K., Kawai T. Signal requirement for interleukin-1-dependent interleukin 2 production by a human leukemia-derived HSB.2 subclone. J Immunol. 1987 Nov 15;139(10):3321–3329. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Okada M., Maeda M., Tagaya Y., Taniguchi Y., Teshigawara K., Yoshiki T., Diamantstein T., Smith K. A., Uchiyama T., Honjo T. TCGF(IL 2)-receptor inducing factor(s). II. Possible role of ATL-derived factor (ADF) on constitutive IL 2 receptor expression of HTLV-I(+) T cell lines. J Immunol. 1985 Dec;135(6):3995–4003. [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi H., Bertoglio J., Tursz T., Fradelizi D. IL 2 receptor induction on human T lymphocytes: role for IL 2 and monocytes. J Immunol. 1985 Jul;135(1):321–327. [PubMed] [Google Scholar]
- Wiskocil R., Weiss A., Imboden J., Kamin-Lewis R., Stobo J. Activation of a human T cell line: a two-stimulus requirement in the pretranslational events involved in the coordinate expression of interleukin 2 and gamma-interferon genes. J Immunol. 1985 Mar;134(3):1599–1603. [PubMed] [Google Scholar]