Abstract
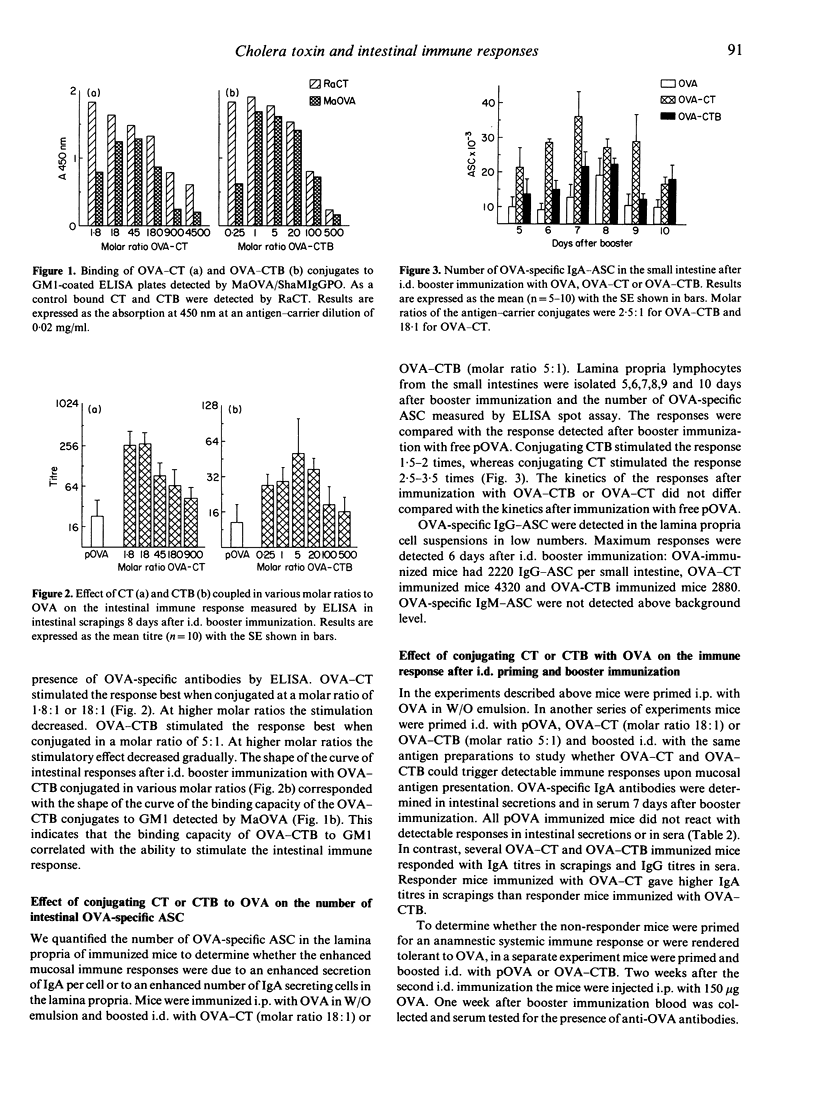
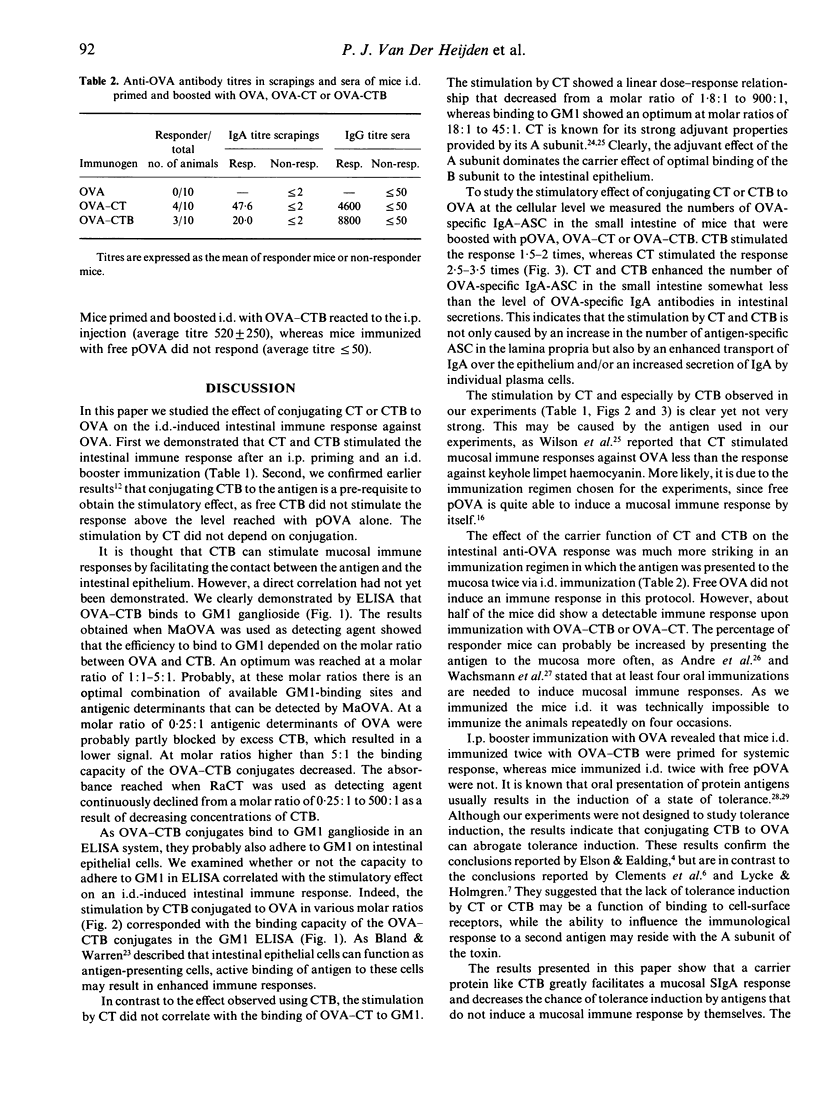
We studied the effect of mucosal presentation of ovalbumin (OVA) conjugated to cholera toxin (CT) or cholera toxin B subunit (CTB) on the intestinal immune responses against OVA. Mice were primed intraperitoneally (i.p.) with OVA in a water-in-oil emulsion and boosted intraduodenally (i.d.) with OVA conjugated to CT or CTB in various molar ratios. Responses were evaluated by testing intestinal secretions for OVA-specific antibodies and by quantifying the OVA-specific antibody secreting cells (ASC) in the lamina propria of the small intestine. OVA-CT conjugates were tested in a molar ratio ranging from 1.8:1 to 4500:1. OVA-CTB conjugates were tested in a molar ratio ranging from 0.25:1 to 500:1. The optimum intestinal immune response was reached at a molar ratio of 1.8:1 for OVA-CT and 5:1 for OVA-CTB. The binding capacity of OVA-CTB, but not of OVA-CT, to GM1 ganglioside corresponded with the capacity to enhance the intestinal immune response. The effect of conjugating CTB or CT to OVA on the immune response against OVA was more striking when mice were not only boosted i.d., but also primed i.d. Both OVA-CT and OVA-CTB induced detectable immune responses, whereas free OVA did not. Therefore, the carrier effect of CT or CTB is essential to trigger a mucosal immune response against OVA when presented mucosally only. We conclude that enhancing antigen uptake greatly facilitates mucosal immune responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., Bazin H., Heremans J. F. Influence of repeated administration of antigen by the oral route on specific antibody-producing cells in the mouse spleen. Digestion. 1973;9(2):166–175. doi: 10.1159/000197442. [DOI] [PubMed] [Google Scholar]
- Bianchi A. T., Scholten J. W., Jongenelen I. M., Koch G. The use of monoclonal antibodies in an enzyme immunospot assay to detect isotype-specific antibody-secreting cells in pigs and chickens. Vet Immunol Immunopathol. 1990 Feb;24(2):125–134. doi: 10.1016/0165-2427(90)90015-k. [DOI] [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. I. Kinetics, antigen specificity and blocking by anti-Ia antisera. Immunology. 1986 May;58(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Hartzog N. M., Lyon F. L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988 Jun;6(3):269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Russell M. W., Lycke N., Lindblad M., Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989 Apr;57(4):1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984 Dec;133(6):2892–2897. [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Arousal of mucosal secretory immunoglobulin A antitoxin in rats immunized with Escherichia coli heat-labile enterotoxin. Infect Immun. 1982 Sep;37(3):1086–1092. doi: 10.1128/iai.37.3.1086-1092.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Cholera toxin as a mucosal adjuvant. Glutaraldehyde treatment dissociates adjuvanticity from toxicity. J Immunol. 1989 Jul 15;143(2):484–490. [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988 Sep 1;141(5):1495–1501. [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedrud J. G., Liang X. P., Hague N., Lamm M. E. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987 Nov 15;139(10):3484–3492. [PubMed] [Google Scholar]
- Parker C. W. Role of cyclic nucleotides in regulating lymphocytes. Ann N Y Acad Sci. 1979;332:255–261. doi: 10.1111/j.1749-6632.1979.tb47119.x. [DOI] [PubMed] [Google Scholar]
- Schödel F., Will H. Construction of a plasmid for expression of foreign epitopes as fusion proteins with subunit B of Escherichia coli heat-labile enterotoxin. Infect Immun. 1989 Apr;57(4):1347–1350. doi: 10.1128/iai.57.4.1347-1350.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Kurata H., Funato H., Nagamine T., Aizawa C., Kurata T. Protection against influenza virus infection by a two-dose regimen of nasal vaccination using vaccines combined with cholera toxin B subunit. Vaccine. 1989 Aug;7(4):314–320. doi: 10.1016/0264-410x(89)90192-8. [DOI] [PubMed] [Google Scholar]
- Tamura S., Samegai Y., Kurata H., Nagamine T., Aizawa C., Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988 Oct;6(5):409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr Oral tolerance. Transplantation. 1980 May;29(5):353–356. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- Van der Heijden P. J., Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J Immunol Methods. 1987 Nov 5;103(2):161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- Wachsmann D., Klein J. P., Schöller M., Frank R. M. Local and systemic immune response to orally administered liposome-associated soluble S. mutans cell wall antigens. Immunology. 1985 Jan;54(1):189–193. [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Stokes C. R., Bourne F. J. Adjuvant effect of cholera toxin on the mucosal immune response to soluble proteins. Differences between mouse strains and protein antigens. Scand J Immunol. 1989 Jun;29(6):739–745. [PubMed] [Google Scholar]


