Abstract
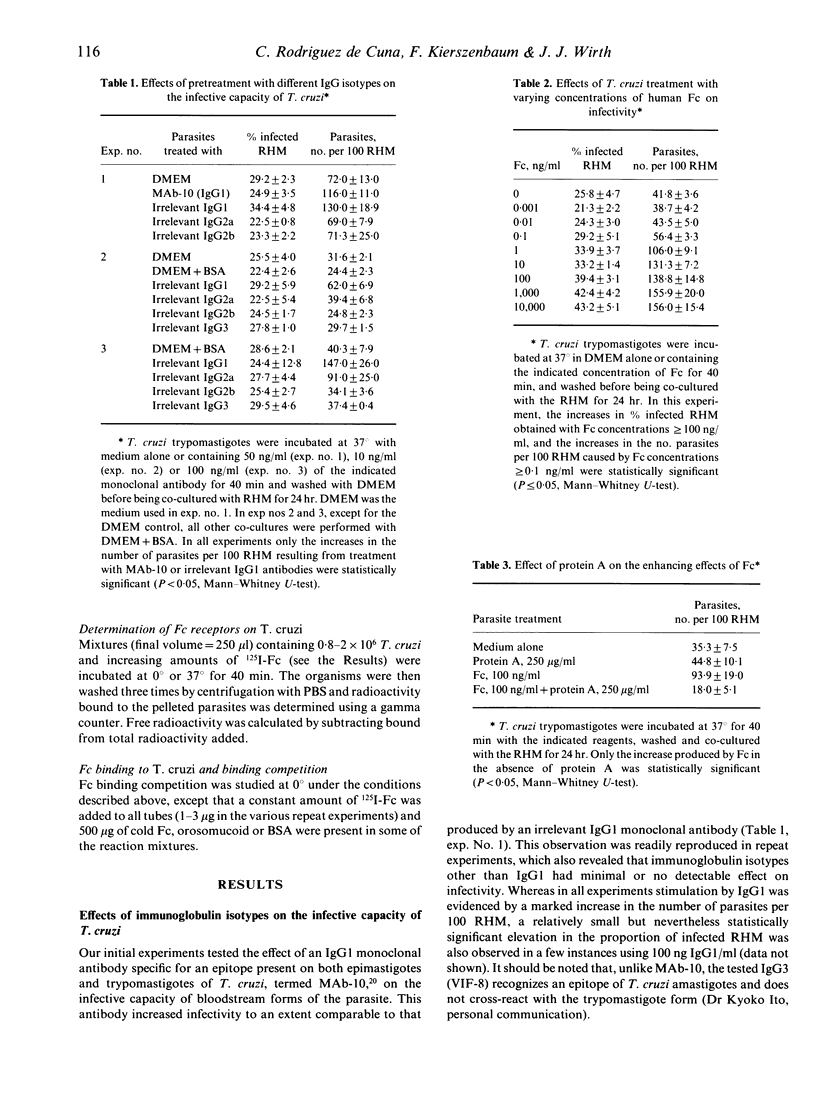
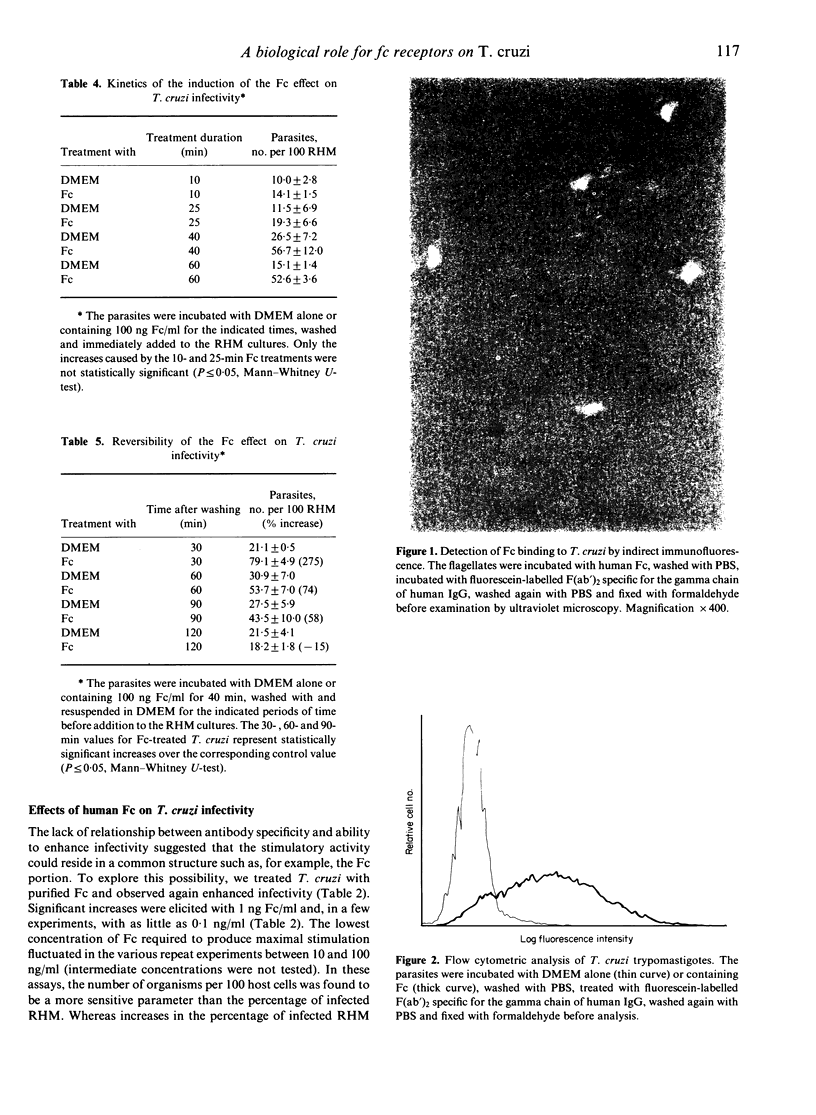
The infective capacity of Trypanosoma cruzi was significantly increased after treatment with monoclonal IgG1 antibodies, whether or not specific for the parasite; minimal or no change in infectivity was seen after treatment with IgG2a, IgG2b or IgG3 monoclonal antibodies. The stimulatory effect was evidenced by elevated numbers of trypanosomes invading mammalian host cells in vitro compared to parasites treated with medium alone. Greater infectivity was also induced by pure human Fc, suggesting a role for Fc receptors on the organism. This inference received support in the fact that protein A inhibited the stimulatory effect of Fc. In addition, Fc-treated parasites incubated with fluorescein-labelled F(ab')2 from goat anti-human IgG exhibited fluorescence detectable by both ultraviolet microscopy and flow cytometry. 125I-Fc binding to T. cruzi was found to be saturable at 0 degrees and was inhibited by cold Fc but not by bovine serum albumin (BSA) or orosomucoid. Interestingly, 125I-Fc binding was greater at 37 degrees and it was not saturable with the concentrations that did saturate at 0 degrees. Possibly, Fc might up-regulate expression of its own receptor and greater endocytosis could take place at 37 degrees. Significant increases in infectivity were detectable after a 40 min pretreatment with Fc--hinting that Fc could trigger a chain of biochemical events underlying the phenomenon--and were reversible, becoming undetectable 2 hr after Fc removal. The average number of Fc receptors per parasite, determined at 0 degrees (at which binding saturation was possible), was estimated as 5 x 10(5), the dissociation constant was of the order of 10(-6)-10(7)M. The present results define an important biological role for an Fc-binding T. cruzi surface component and expose the capacity of this organism to exploit even elements of the immune system in its quest to attain intracellular localization, required for multiplication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcina A., Fresno M. A tubulin-related 55 kilodalton surface antigen recognized by different Trypanosoma cruzi stage-specific monoclonal antibodies from infected mice. Mol Biochem Parasitol. 1988 Jun;29(2-3):181–190. doi: 10.1016/0166-6851(88)90073-4. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Kierszenbaum F. Isolation of Trypanosoma cruzi from blood. J Parasitol. 1974 Dec;60(6):1037–1038. [PubMed] [Google Scholar]
- Budzko D. B., Pizzimenti M. C., Kierszenbaum F. Effects of complement depletion in experimental chagas disease: immune lysis of virulent blood forms of Trypanosoma cruzi. Infect Immun. 1975 Jan;11(1):86–91. doi: 10.1128/iai.11.1.86-91.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M. C., Ayala A., Kierszenbaum F. Effects of alpha- and beta-adrenergic agonists on Trypanosoma cruzi interaction with host cells. J Parasitol. 1988 Jun;74(3):379–386. [PubMed] [Google Scholar]
- De Miranda-Santos I. K., Campos-Neto A. Receptor for immunoglobulin Fc on pathogenic but not on nonpathogenic protozoa of the Trypanosomatidae. J Exp Med. 1981 Dec 1;154(6):1732–1742. doi: 10.1084/jem.154.6.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M. M., Kierszenbaum F. Experimental Chagas' disease: kinetics of lymphocyte responses and immunological control of the transition from acute to chronic Trypanosoma cruzi infection. Infect Immun. 1981 Mar;31(3):1117–1124. doi: 10.1128/iai.31.3.1117-1124.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F. Antibody-dependent killing of bloodstream forms of Trypanosoma cruzi by human peripheral blood leukocytes. Am J Trop Med Hyg. 1979 Nov;28(6):965–968. doi: 10.4269/ajtmh.1979.28.965. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Howard J. G. Mechanisms of resistance against experimental Trypanosoma cruzi infection: the importance of antibodies and antibody-forming capacity in the Biozzi high and low responder mice. J Immunol. 1976 May;116(5):1208–1211. [PubMed] [Google Scholar]
- Kierszenbaum F., Lima M. F., Wirth J. J. Effects of antiserum to Trypanosoma cruzi on the uptake and rate of killing of vector-borne, metacyclic forms of the parasite by macrophages. Int J Parasitol. 1985 Aug;15(4):409–413. doi: 10.1016/0020-7519(85)90026-8. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Wirth J. J., McCann P. P., Sjoerdsma A. Arginine decarboxylase inhibitors reduce the capacity of Trypanosoma cruzi to infect and multiply in mammalian host cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4278–4282. doi: 10.1073/pnas.84.12.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis T. L., James S. L., Sher A., David J. R. Cell-mediated cytotoxicity to Trypanosoma cruzi. II. Antibody-dependent killing of bloodstream forms by mouse eosinophils and neutrophils. Am J Trop Med Hyg. 1981 Jan;30(1):47–53. [PubMed] [Google Scholar]
- Kirchhoff L. V., Engel J. C., Dvorak J. A., Sher A. Strains and clones of Trypanosoma cruzi differ in their expression of a surface antigen identified by a monoclonal antibody. Mol Biochem Parasitol. 1984 Apr;11:81–89. doi: 10.1016/0166-6851(84)90056-2. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976 Mar;116(3):755–760. [PubMed] [Google Scholar]
- Krettli A. U., Weisz-Carrington P., Nussenzweig R. S. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin Exp Immunol. 1979 Sep;37(3):416–423. [PMC free article] [PubMed] [Google Scholar]
- Mercado T. I., Katusha K. Isolation of Trypanosoma cruzi from the blood of infected mice by column chromatography. Prep Biochem. 1979;9(1):97–106. doi: 10.1080/00327487908061675. [DOI] [PubMed] [Google Scholar]
- Mocelin A. J., Brandina L., Gordan P. A., Baldy J. L., Chieffi P. P. Immunosuppression and circulating Trypanosoma cruzi in a kidney transplant recipient. Transplantation. 1977 Feb;23(2):163–163. doi: 10.1097/00007890-197702000-00011. [DOI] [PubMed] [Google Scholar]
- Norris K. A., Harth G., So M. Purification of a Trypanosoma cruzi membrane glycoprotein which elicits lytic antibodies. Infect Immun. 1989 Aug;57(8):2372–2377. doi: 10.1128/iai.57.8.2372-2377.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G., Douglass T. G., Speer C. A. Surface interactions between macrophages and Trypanosoma cruzi. Am J Trop Med Hyg. 1982 Jul;31(4):723–729. doi: 10.4269/ajtmh.1982.31.723. [DOI] [PubMed] [Google Scholar]
- Rodriguez A. M., Santoro F., Afchain D., Bazin H., Capron A. Trypanosoma cruzi infection in B-cell-deficient rats. Infect Immun. 1981 Feb;31(2):524–529. doi: 10.1128/iai.31.2.524-529.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snary D., Hudson L. Trypanosoma cruzi cell surface proteins: identification of one major glycoprotein. FEBS Lett. 1979 Apr 1;100(1):166–170. doi: 10.1016/0014-5793(79)81156-4. [DOI] [PubMed] [Google Scholar]
- Takle G. B., Young A., Snary D., Hudson L., Nicholls S. C. Cloning and expression of a trypomastigote-specific 85-kilodalton surface antigen gene from Trypanosoma cruzi. Mol Biochem Parasitol. 1989 Nov;37(1):57–64. doi: 10.1016/0166-6851(89)90102-3. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Eisen H. Fl-160. A surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J Exp Med. 1989 Mar 1;169(3):641–652. doi: 10.1084/jem.169.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Inhibition of mammalian host cell infection by insect-derived, metacyclic forms of Trypanosoma cruzi in the presence of human or rabbit anti-T. cruzi antibodies. Int J Parasitol. 1987 Feb;17(3):841–845. doi: 10.1016/0020-7519(87)90067-1. [DOI] [PubMed] [Google Scholar]