Abstract
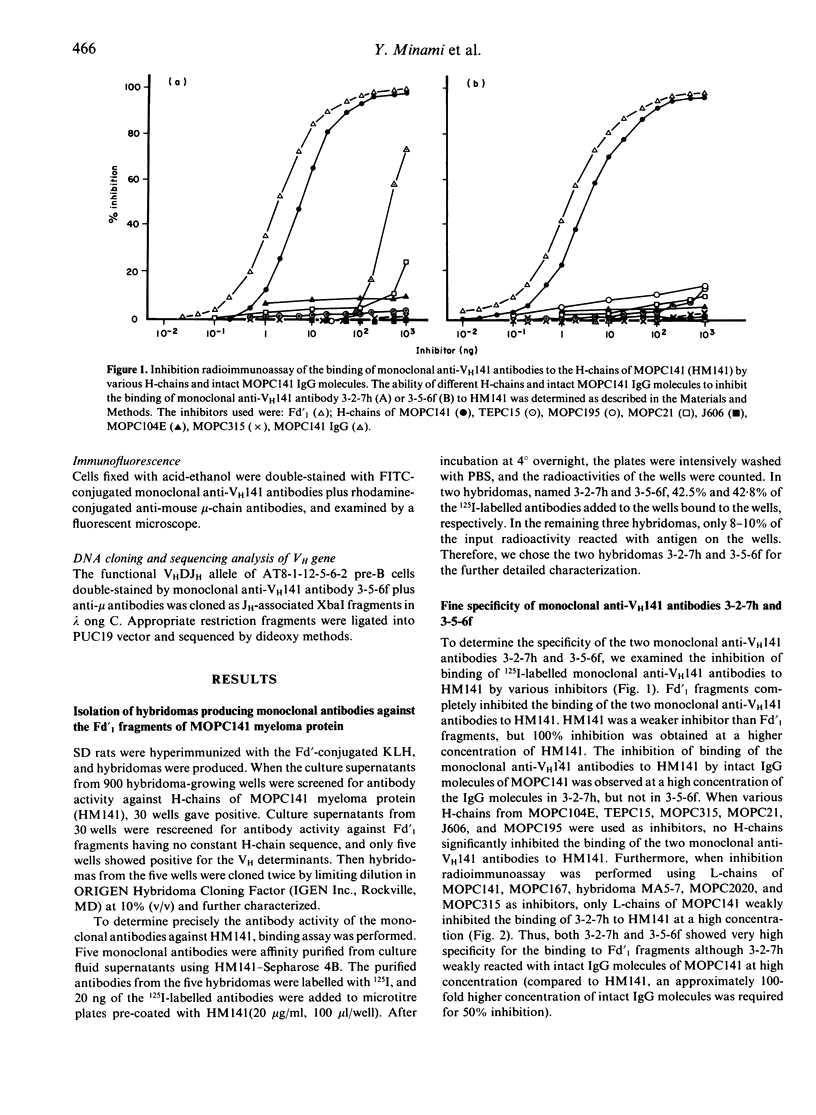
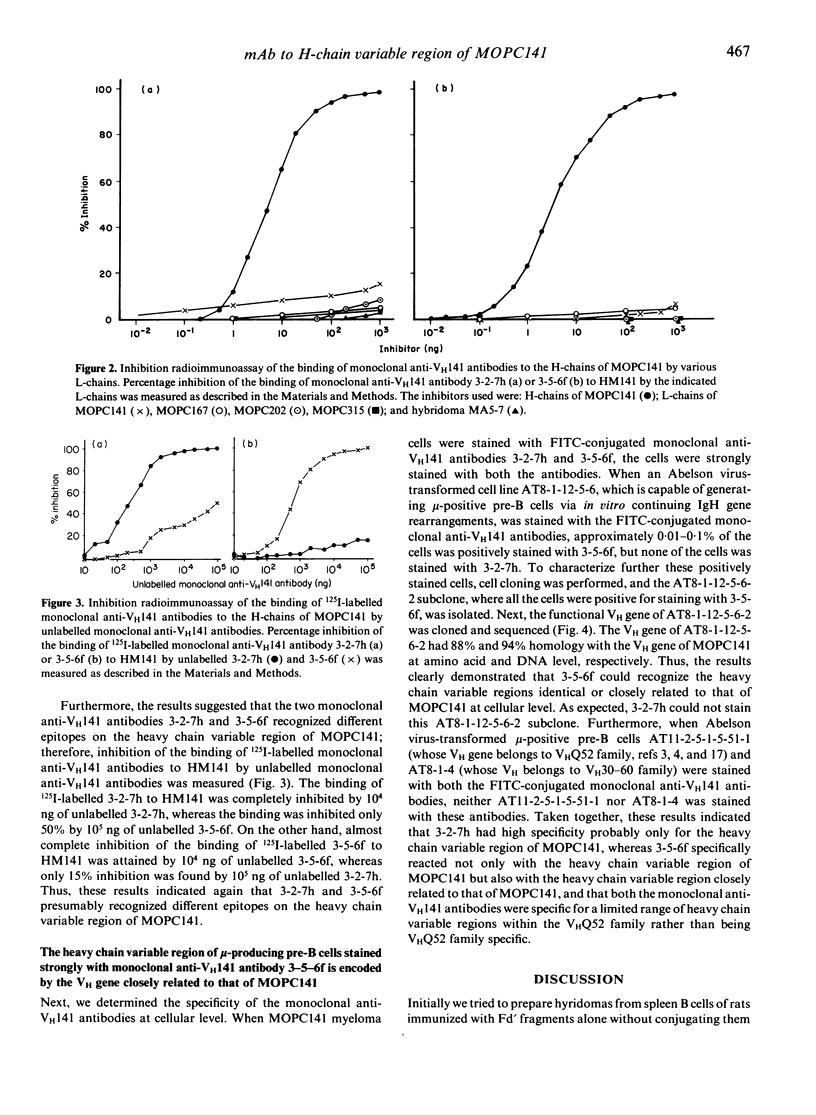
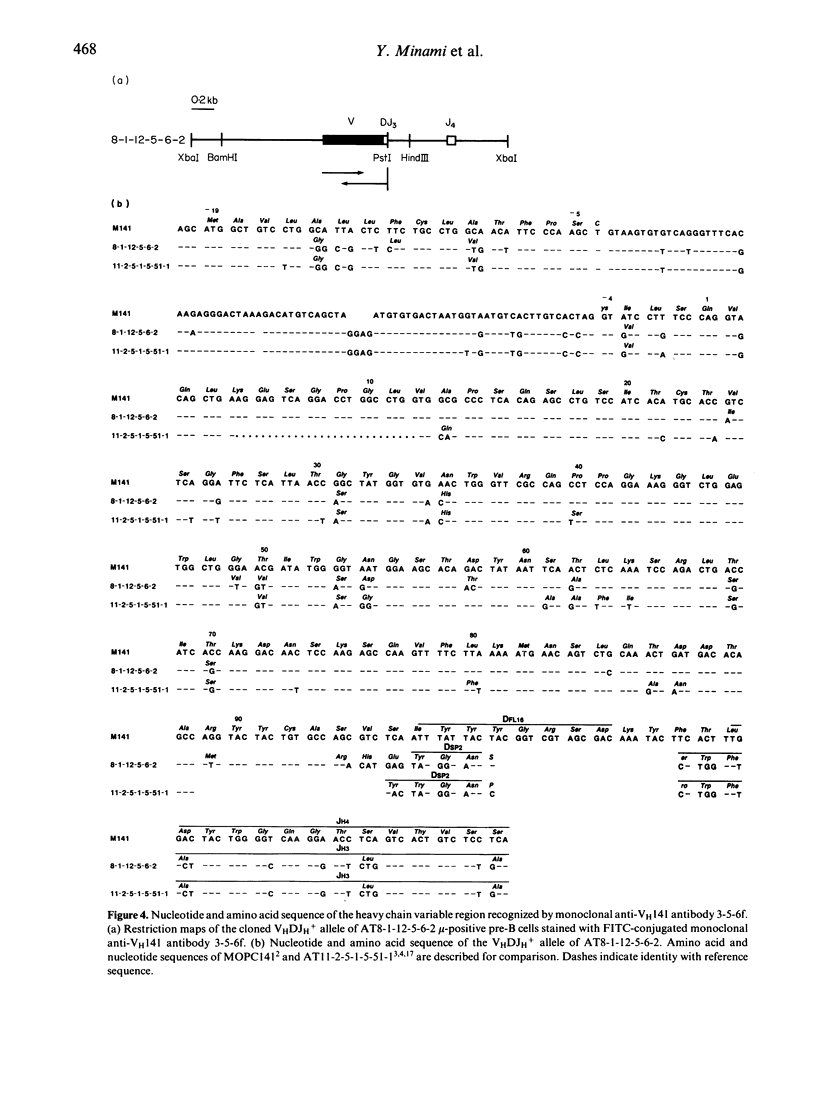
To raise monoclonal antibodies that specifically recognize the heavy chain variable region of MOPC141 myeloma protein (VH141), which belongs to VHQ52 family, rats were immunized with Fd'-conjugated keyhole limpet haemocyanin (KLH) (Fd': Fd' fragments of MOPC141), and the spleen cells were fused with mouse myeloma cells. The resulting 900 hybridomas were screened for antibody activity against Fd'1 fragments having no constant H-chain sequences, which were prepared by cleavage of the Fd' fragments with cyanogen bromide, and two monoclonal antibodies, designated 3-2-7h and 3-5-6f, were obtained. Radioimmunoassay inhibition test showed that the two monoclonal antibodies specifically recognized the VH141, but each was directed to a different determinant on the VH141. When the functional VH gene of Abelson virus-transformed mu-producing pre-B cells, which could be strongly stained with 3-5-6f monoclonal antibody, was cloned and sequenced, the VH gene was closely relate to that of MOPC141 (88% and 94% homology at amino acid and DNA level, respectively). Taken together, the results indicated that 3-2-7h had high specificity only for the VH141, whereas 3-5-6f specifically reacted not only with the VH141 but also with the VH region closely related to that of MOPC141, and that both the monoclonal anti-VH141 antibodies were specific for a limited range of VH regions within the VHQ52 family rather than being VHQ52 family specific. These monoclonal anti-VH141 antibodies should be very useful to determine at a single cell level by immunofluorescence the usage of the VH gene(s) identical or closely related to that of MOPC141 during early B-cell development.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y., Wuilmart C., Lonai P., Givol D. Preparation and characterization of anti-framework antibodies to the heavy chain variable region (VH) of mouse immunoglobulins. Eur J Immunol. 1978 Nov;8(11):797–801. doi: 10.1002/eji.1830081109. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Eshhar Z., Gigi O., Givol D., Ben-Neriah Y. Monoclonal anti-VH antibodies recognize a common VH determinant expressed on immunoglobulin heavy chains from various species. Eur J Immunol. 1983 Jul;13(7):533–540. doi: 10.1002/eji.1830130704. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Hiramoto R., Ghanta V. K., McGhee J. R., Schrohenloher R., Hamlin N. M. Use of dextran conjugated columns for the isolation of large quantities of MOPC 104E IgM. Immunochemistry. 1972 Dec;9(12):1251–1253. doi: 10.1016/0019-2791(72)90300-x. [DOI] [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- Komori T., Sugiyama H., Kishimoto S. A novel VHDJH to JH joining that induces H chain production in an Ig-null immature B cell line. J Immunol. 1989 Aug 1;143(3):1040–1045. [PubMed] [Google Scholar]
- Krawinkel U., Christoph T., Blankenstein T. Organization of the Ig VH locus in mice and humans. Immunol Today. 1989 Oct;10(10):339–344. doi: 10.1016/0167-5699(89)90191-6. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Mayumi M., Kearney J. F., Cooper M. D. Immunoglobulin VH determinants defined by monoclonal antibodies. J Exp Med. 1982 Oct 1;156(4):1010–1024. doi: 10.1084/jem.156.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Sugiyama H., Tani Y., Kishimoto S. The DJH complex remains active in recombination to VH segments after the loss of mu-chain expression in mu-positive pre-B cells. J Immunol. 1989 May 15;142(10):3652–3656. [PubMed] [Google Scholar]
- Maeda T., Sugiyama H., Tani Y., Miyake S., Oka Y., Ogawa H., Komori T., Soma T., Kishimoto S. Start of mu-chain production by the further two-step rearrangements of immunoglobulin heavy chain genes on one chromosome from a DJH/DJH configuration in an Abelson virus-transformed cell line: evidence of secondary DJH complex formation. J Immunol. 1987 Apr 1;138(7):2305–2310. [PubMed] [Google Scholar]
- Oka Y., Sugiyama H., Tsukada S., Kishimoto S. Immature B cells can pass through a VHDJH/germ line state in the Ig H chain gene rearrangements. J Immunol. 1990 Jul 1;145(1):361–364. [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Jackson S., Alt F. W. VHDJH formation and DJH replacement during pre-B differentiation: non-random usage of gene segments. EMBO J. 1986 Sep;5(9):2131–2138. doi: 10.1002/j.1460-2075.1986.tb04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Gehrmann P., Petrac E., Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. 1986 Aug 28-Sep 3Nature. 322(6082):840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sakato N., Eisen H. N. Antibodies to idiotypes of isologous immunoglobulins. J Exp Med. 1975 Jun 1;141(6):1411–1426. doi: 10.1084/jem.141.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakato N., Semma M., Eisen H. N., Azuma T. A small hypervariable segment in the variable domain of an immunoglobulin light chain stimulates formation of anti-idiotypic suppressor T cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5396–5400. doi: 10.1073/pnas.79.17.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Akira S., Kikutani H., Kishimoto S., Yamamura Y., Kishimoto T. Functional V region formation during in vitro culture of a murine immature B precursor cell line. Nature. 1983 Jun 30;303(5920):812–815. doi: 10.1038/303812a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Maeda T., Tani Y., Miyake S., Oka Y., Komori T., Ogawa H., Soma T., Minami Y., Sakato N. Selective use of the VHQ52 family in functional VH to DJH rearrangements in a B precursor cell line. J Exp Med. 1987 Aug 1;166(2):607–612. doi: 10.1084/jem.166.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H., Minami Y., Komori T., Sakato N., Kishimoto S. Infrequent utilization of the immunoglobulin heavy chain variable region(s) identical or closely related to that of MOPC315 myeloma protein in the functional V region formation in B-precursor cell lines. Immunology. 1989 Dec;68(4):453–457. [PMC free article] [PubMed] [Google Scholar]
- Vrana M., Tomasić J., Glaudemans C. P. Purification of homogeneous murine immunoglobulins with anti-fructofuranan specificity. J Immunol. 1976 Jun;116(6):1662–1663. [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Matsunami N., Choi C. Y., Sugiyama H., Kishimoto T., Honjo T. The D-JH complex is an intermediate to the complete immunoglobulin heavy-chain V-region gene. Nucleic Acids Res. 1983 Nov 11;11(21):7303–7316. doi: 10.1093/nar/11.21.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]


