Abstract
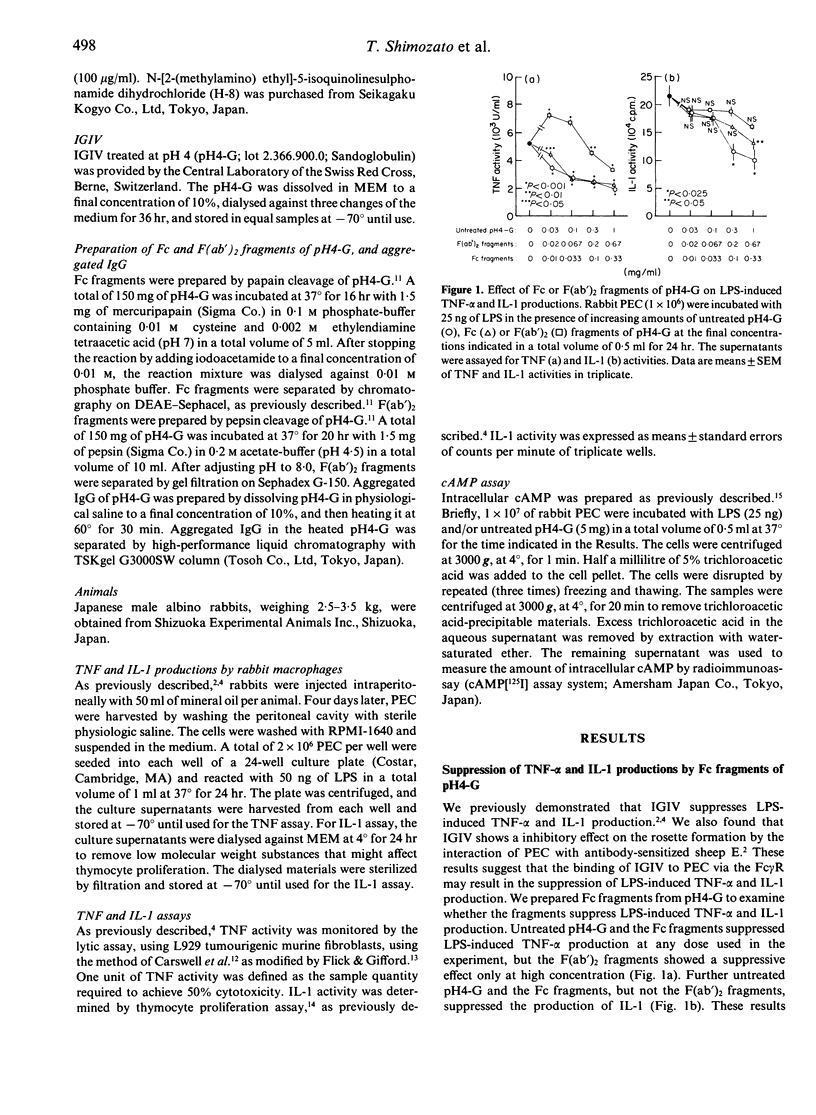
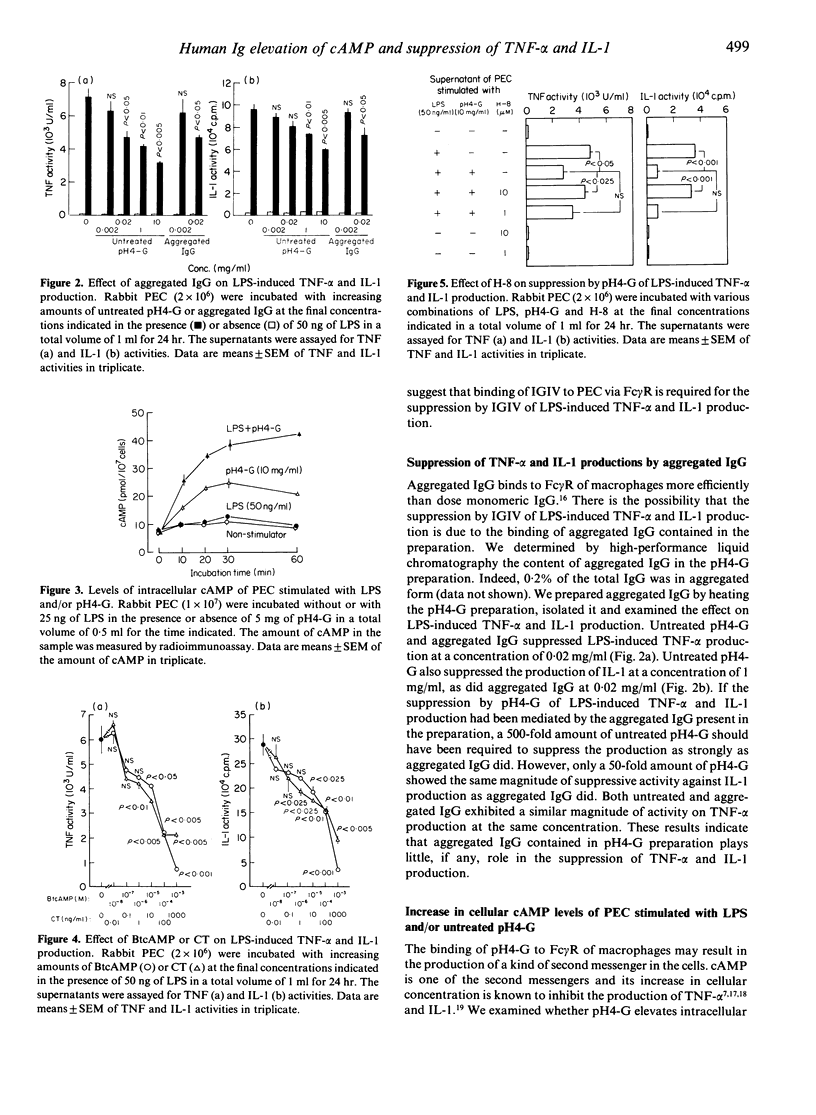
We previously showed that human immunoglobulin preparation for intravenous use (IGIV) suppresses the in vitro production of tumour necrosis factor-alpha (TNF-alpha) and interleukin-1 (IL-1) by rabbit peritoneal exudate cells (PEC) stimulated with lipopolysaccharide (LPS). In this study we investigated the mechanism of the suppression. IGIV treated at pH4 (pH4-G) was used as IGIV. Fc fragments of pH4-G, as well as untreated pH4-G, suppressed TNF-alpha and IL-1 production by rabbit PEC stimulated with LPS. The interaction of pH4-G with PEC also resulted in generation of cyclic adenosine 3':5'-monophosphate (cAMP), known to be an intracellular second messenger. N6, 2'-0-dibutyryl cAMP (BtcAMP), a lipid-soluble derivative of cAMP, and cholera toxin (CT), an adenylate cyclase activating agent, also suppressed the production of TNF-alpha and IL-1. Further N-[2-(methylamino) ethyl]-5-isoquinolinesulphonamide dihydrochloride (H-8), an inhibitor of cAMP-dependent protein kinases, abrogated the suppression by pH4-G of the productions. These results indicate that the binding of IGIV to PEC via Fc gamma receptors (Fc gamma R) induces the elevation of intracellular cAMP levels, resulting in the suppression of LPS-induced TNF-alpha and IL-1 productions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandwein S. R. Regulation of interleukin 1 production by mouse peritoneal macrophages. Effects of arachidonic acid metabolites, cyclic nucleotides, and interferons. J Biol Chem. 1986 Jul 5;261(19):8624–8632. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Baeder W., Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Gemsa D., Steggemann L., Menzel J., Till G. Release of cyclic AMP from macrophages by stimulation with prostaglandins. J Immunol. 1975 Apr;114(4):1422–1424. [PubMed] [Google Scholar]
- Hart P. H., Whitty G. A., Piccoli D. S., Hamilton J. A. Control by IFN-gamma and PGE2 of TNF alpha and IL-1 production by human monocytes. Immunology. 1989 Mar;66(3):376–383. [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Iwata M., Shimozato T., Tokiwa H., Tsubura E. Antipyretic activity of a human immunoglobulin preparation for intravenous use in an experimental model of fever in rabbits. Infect Immun. 1987 Mar;55(3):547–554. doi: 10.1128/iai.55.3.547-554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakami Y., Nakao Y., Koizumi T., Katakami N., Ogawa R., Fujita T. Regulation of tumour necrosis factor production by mouse peritoneal macrophages: the role of cellular cyclic AMP. Immunology. 1988 Aug;64(4):719–724. [PMC free article] [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Kunkel S. L., Wiggins R. C., Chensue S. W., Larrick J. Regulation of macrophage tumor necrosis factor production by prostaglandin E2. Biochem Biophys Res Commun. 1986 May 29;137(1):404–410. doi: 10.1016/0006-291x(86)91224-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Masaoka T., Nakamura T., Shimada K., Urushizaki I., Kogo Y., Saito A., Tomizawa M., Nakayana I., Mikuni C. [Therapeutic evaluation of combination therapy with IG-100, pH4-treated human immunoglobulin preparation for i.v. administration, and antibiotics against severe infections. The Society for Clinical Investigation on IG-100]. Kansenshogaku Zasshi. 1984 Oct;58(10):1001–1024. doi: 10.11150/kansenshogakuzasshi1970.58.1001. [DOI] [PubMed] [Google Scholar]
- Morgan E. L., Weigle W. O. Biological activities residing in the Fc region of immunoglobulin. Adv Immunol. 1987;40:61–134. doi: 10.1016/s0065-2776(08)60238-2. [DOI] [PubMed] [Google Scholar]
- Nitta T., Suzuki T. Biochemical signals transmitted by Fc gamma receptors: triggering mechanisms of the increased synthesis of adenosine-3',5'-cyclic monophosphate mediated by Fc gamma 2a- and Fc gamma 2b- -receptors of a murine macrophage-like cell line (P388D1). J Immunol. 1982 Dec;129(6):2708–2714. [PubMed] [Google Scholar]
- Onozaki K., Tamatani T., Hashimoto T., Matsushima K. Growth inhibition and augmentation of mouse myeloid leukemic cell line differentiation by interleukin 1. Cancer Res. 1987 May 1;47(9):2397–2402. [PubMed] [Google Scholar]
- Renz H., Gong J. H., Schmidt A., Nain M., Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct 1;141(7):2388–2393. [PubMed] [Google Scholar]
- Shimozato T., Iwata M., Tamura N. Suppression of tumor necrosis factor alpha production by a human immunoglobulin preparation for intravenous use. Infect Immun. 1990 May;58(5):1384–1390. doi: 10.1128/iai.58.5.1384-1390.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Remick D. G., Ward P. A., Spengler R. N., Lynch J. P., 3rd, Larrick J., Kunkel S. L. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]



