Abstract
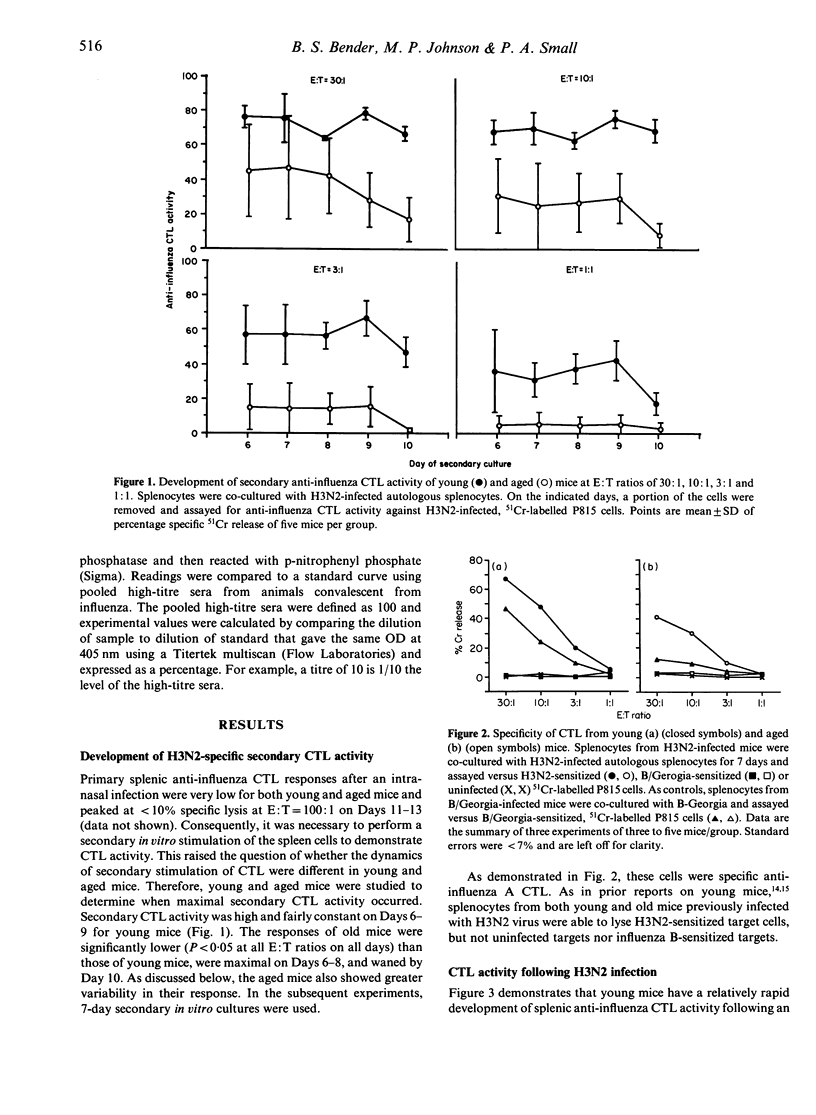
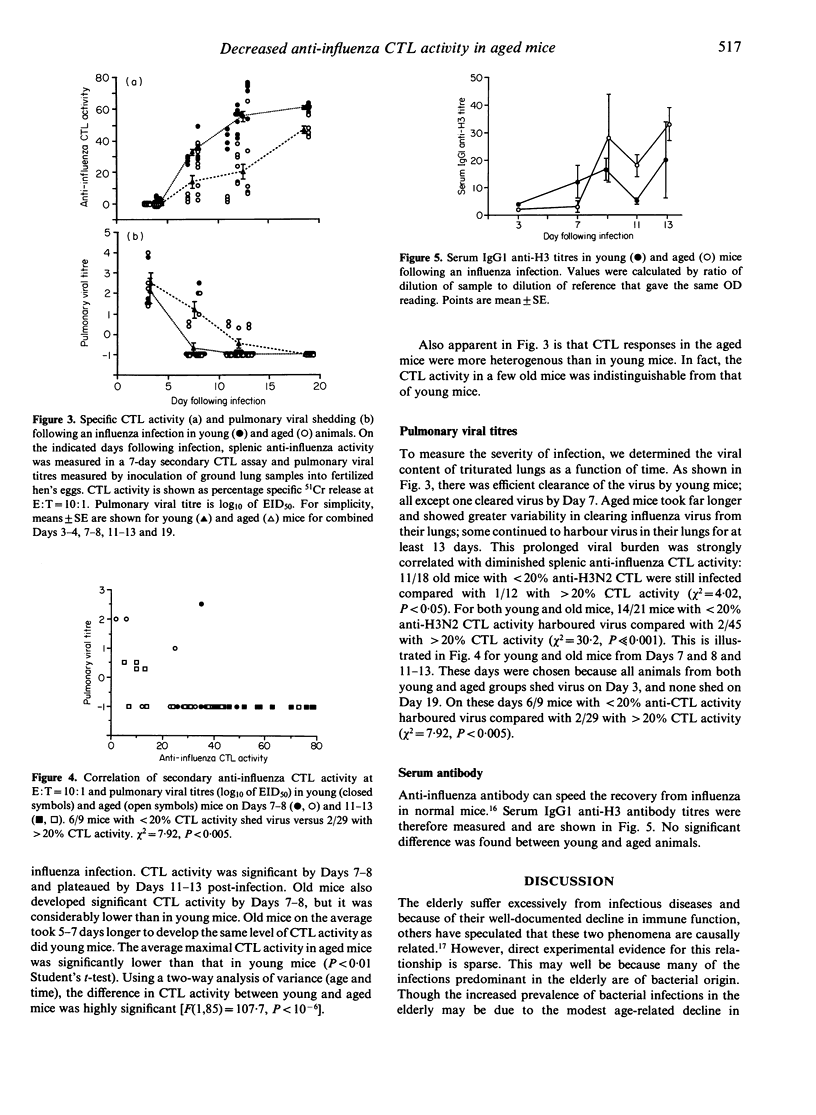
Influenza and pneumonia are leading causes of death in the elderly. Cytotoxic T-lymphocyte activity is responsible for viral clearance after infection and declines with age. We hypothesized that following intranasal infection with influenza virus, aged mice would have decreased anti-influenza cytotoxic T-lymphocyte activity that would correlate with prolonged pulmonary viral shedding. To test this, young (1.5-4.0 month) and aged (22-25 month) BALB/c mice were infected intranasally with influenza A/Port Chalmers/1/73(H3N2). Mice were killed at 3-19 days following infection. Their splenic cytotoxic T-lymphocyte activity was measured by a secondary in vitro chromium release assay. Pulmonary viral titres were quantified by growth of titrated lung specimens in fertilized hens' eggs. Serum antibody titres were measured by an ELISA. Young mice responded in a relatively homogeneous fashion. They developed maximal cytotoxic T-lymphocyte activity of 60.9 +/- 2.0% by Days 11-13, and all except one cleared virus from the lung by Day 7. In contrast, old mice were heterogeneous. Their cytotoxic T-lymphocyte activity peaked at 46.9 +/- 5.0% and was delayed by 5-7 days. Forty-five per cent were still shedding virus at Days 7 and 8, and shedding persisted for at least 13 days in some mice. There was a strong correlation in both young and aged mice between the presence of virus in the lungs and decreased splenic cytotoxic T-lymphocyte activity (chi 2 = 30.2, P much less than 0.001). No significant difference was found between young and aged animals in serum IgG1 anti-H3 antibody titres. We conclude that following influenza infection in aged mice, impaired cytotoxic T-lymphocyte activity leads to prolonged duration of infection. These observations may lead to a better understanding of the excess morbidity and mortality in elderly persons that occur with influenza.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B. Persistence of cell-mediated immunity to influenza A virus in mice. Immunology. 1982 Sep;47(1):165–168. [PMC free article] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moss B. Recognition of cloned influenza virus hemagglutinin gene products by cytotoxic T lymphocytes. J Virol. 1986 Mar;57(3):786–791. doi: 10.1128/jvi.57.3.786-791.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Effros R. B., Bennink J. Heterogeneity of the cytotoxic response of thymus-derived lymphocytes after immunization with influenza viruses. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1209–1213. doi: 10.1073/pnas.74.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager K. B., Hackett C. J., Gerhard W. U., Bennink J., Eisenlohr L. C., Yewdell J., Ricciardi R. P. Murine cell lines stably expressing the influenza virus hemagglutinin gene introduced by a recombinant retrovirus vector are constitutive targets for MHC class I- and class II-restricted T lymphocytes. J Immunol. 1989 Oct 1;143(7):2328–2335. [PubMed] [Google Scholar]
- Effros R. B., Walford R. L. Diminished T-cell response to influenza virus in aged mice. Immunology. 1983 Jun;49(2):387–392. [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Walford R. L. The effect of age on the antigen-presenting mechanism in limiting dilution precursor cell frequency analysis. Cell Immunol. 1984 Oct 15;88(2):531–539. doi: 10.1016/0008-8749(84)90184-9. [DOI] [PubMed] [Google Scholar]
- Effros R. B., Walford R. L. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983 Oct 15;81(2):298–305. doi: 10.1016/0008-8749(83)90237-x. [DOI] [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Age-related decline in the resistance of mice to infection with intracellular pathogens. Infect Immun. 1977 May;16(2):593–598. doi: 10.1128/iai.16.2.593-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioe C. E., Hinshaw V. S. Induction and activity of class II-restricted, Lyt-2+ cytolytic T lymphocytes specific for the influenza H5 hemagglutinin. J Immunol. 1989 Apr 1;142(7):2482–2488. [PubMed] [Google Scholar]
- Kris R. M., Asofsky R., Evans C. B., Small P. A., Jr Protection and recovery in influenza virus-infected mice immunosuppressed with anti-IgM. J Immunol. 1985 Feb;134(2):1230–1235. [PubMed] [Google Scholar]
- Kris R. M., Yetter R. A., Cogliano R., Ramphal R., Small P. A. Passive serum antibody causes temporary recovery from influenza virus infection of the nose, trachea and lung of nude mice. Immunology. 1988 Mar;63(3):349–353. [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louria D. B., Sen P., Buse M. Age-dependent differences in outcome of infections, with special reference to experiments in mice. J Am Geriatr Soc. 1982 Dec;30(12):769–773. doi: 10.1111/j.1532-5415.1982.tb03368.x. [DOI] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur U., Bentley D. W., Hall C. B., Roth F. K., Douglas R. G., Jr Influenza A/Brazil/78(H1N1) infection in the elderly. Am Rev Respir Dis. 1981 Jun;123(6):633–635. doi: 10.1164/arrd.1981.123.6.633. [DOI] [PubMed] [Google Scholar]
- Nagel J. E., Han K., Coon P. J., Adler W. H., Bender B. S. Age differences in phagocytosis by polymorphonuclear leukocytes measured by flow cytometry. J Leukoc Biol. 1986 Apr;39(4):399–407. doi: 10.1002/jlb.39.4.399. [DOI] [PubMed] [Google Scholar]
- Orme I. M. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am Rev Respir Dis. 1988 Mar;137(3):716–718. doi: 10.1164/ajrccm/137.3.716. [DOI] [PubMed] [Google Scholar]
- Patel P. J. Aging and antimicrobial immunity. Impaired production of mediator T cells as a basis for the decreased resistance of senescent mice to listeriosis. J Exp Med. 1981 Sep 1;154(3):821–831. doi: 10.1084/jem.154.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Cogliano R. C., Shands J. W., Jr, Small P. A., Jr Serum antibody prevents lethal murine influenza pneumonitis but not tracheitis. Infect Immun. 1979 Sep;25(3):992–997. doi: 10.1128/iai.25.3.992-997.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N. Class II antigen-specific murine cytolytic T lymphocytes (CTL). I. Analysis of bulk populations and establishment of Lyt-2+L3T4- and Lyt-2-L3T4+ bulk CTL lines. Cell Immunol. 1987 Jul;107(2):395–407. doi: 10.1016/0008-8749(87)90247-4. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Hozumi N., Watanabe M., Bluestone J. A., Johnson-Leva R., Sachs D. H. Class II antigen-specific murine cytolytic T lymphocytes (CTL). II. Genuine class II specificity of Lyt-2+ CTL clones. J Immunol. 1988 Jan 1;140(1):30–36. [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. The cellular and subcellular bases of immunosenescence. Adv Immunol. 1989;46:221–261. doi: 10.1016/s0065-2776(08)60655-0. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Ennis F. A., Albrecht P. Recovery from a viral respiratory infection. II. Passive transfer of immune spleen cells to mice with influenza pneumonia. J Immunol. 1981 Mar;126(3):1042–1046. [PubMed] [Google Scholar]
- Yap K. L., Ada G. L. Cytotoxic T cells in the lungs of mice infected with an influenza A virus. Scand J Immunol. 1978;7(1):73–80. doi: 10.1111/j.1365-3083.1978.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Yetter R. A., Lehrer S., Ramphal R., Small P. A., Jr Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun. 1980 Aug;29(2):654–662. doi: 10.1128/iai.29.2.654-662.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharhary D., Klinman N. R. B cell repertoire diversity to PR8 influenza virus does not decrease with age. J Immunol. 1984 Nov;133(5):2285–2287. [PubMed] [Google Scholar]
- Zweerink H. J., Courtneidge S. A., Skehel J. J., Crumpton M. J., Askonas B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977 May 26;267(5609):354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]


