Abstract
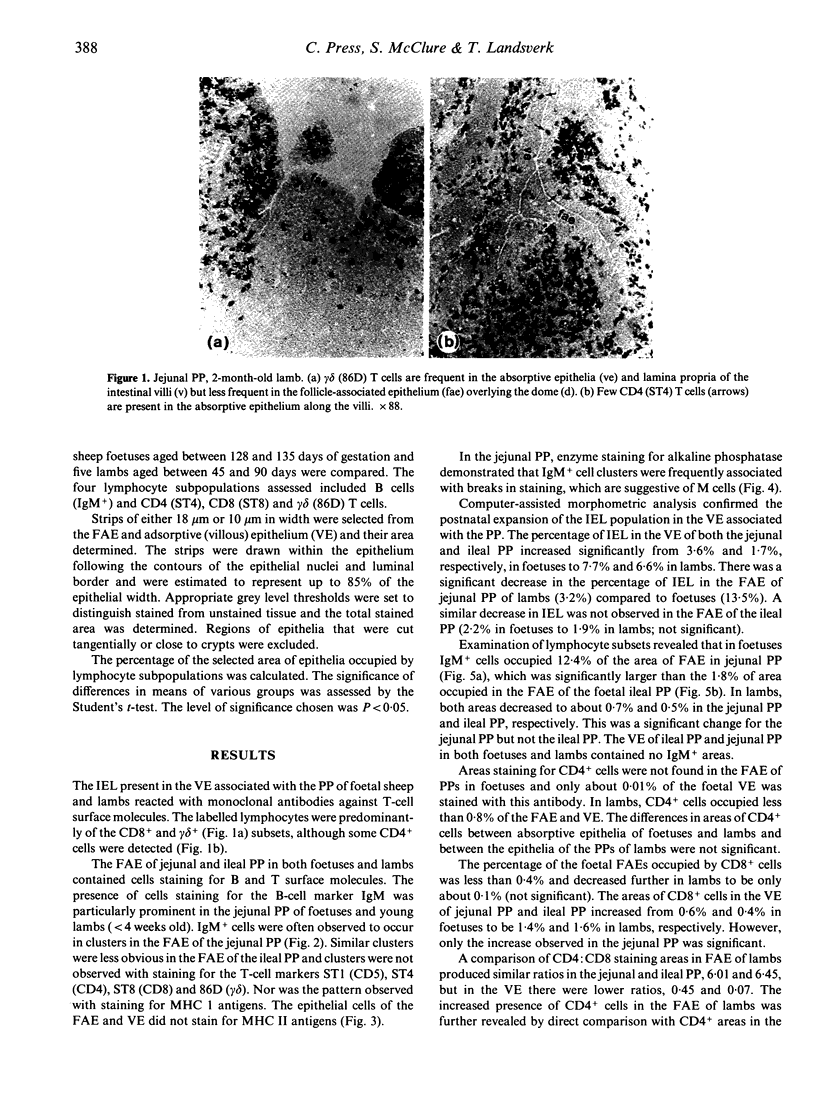
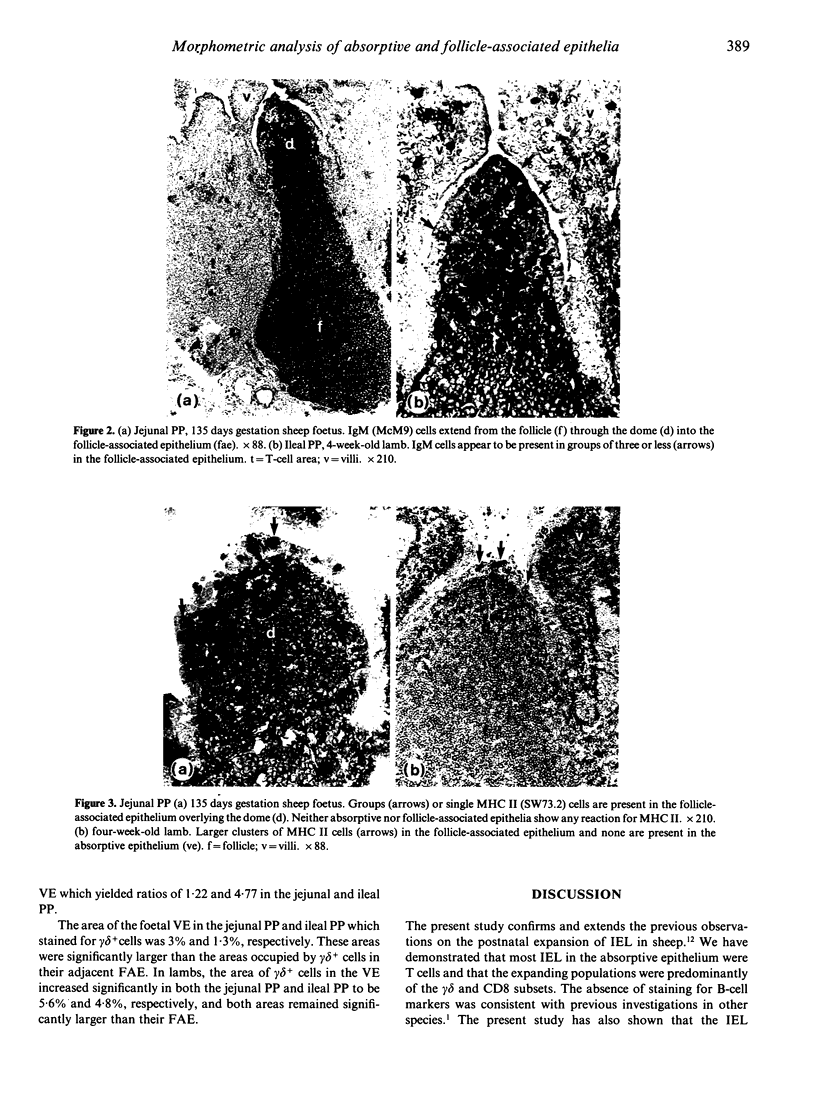
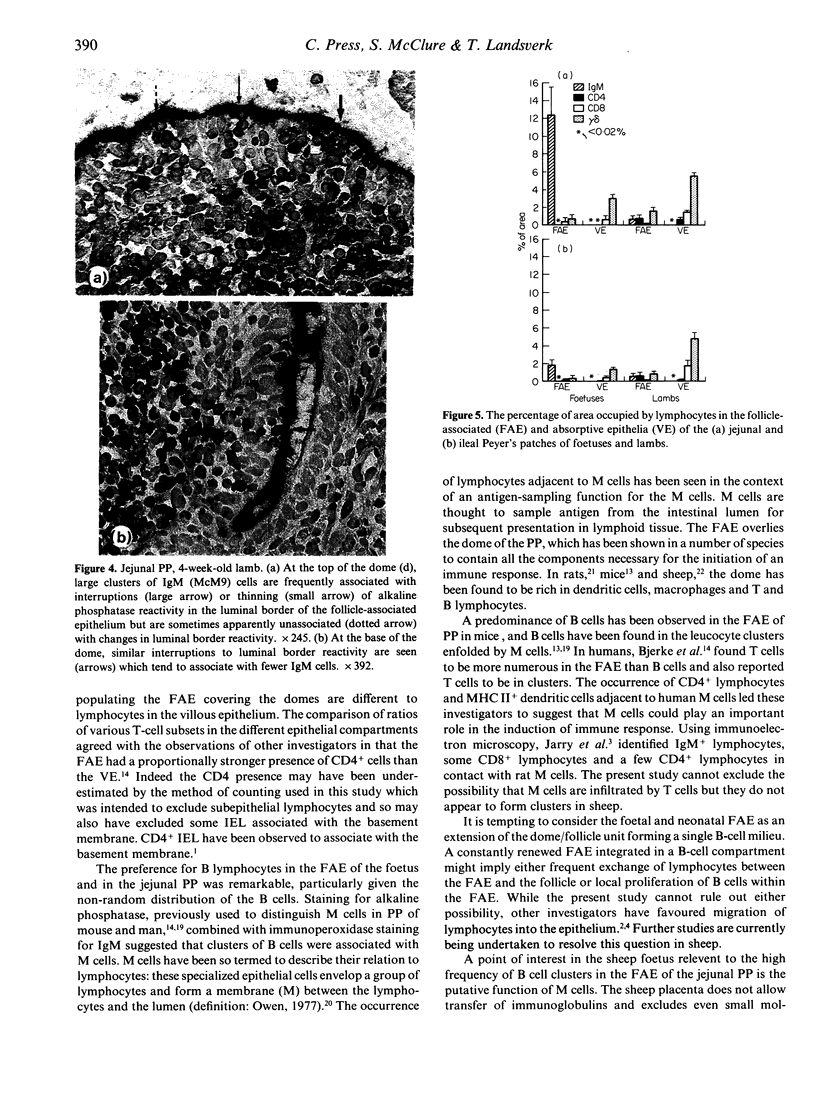
The phenotypes of lymphocytes infiltrating the epithelium of the jejunal and ileal Peyer's patches in foetal sheep at about 130 days gestation and 2-month-old lambs were examined using indirect immunoperoxidase histochemistry, a panel of monoclonal antibodies and enzyme histochemistry. Computer-assisted morphometric analysis enabled the relative size of reactive areas within epithelia to be estimated. The comparison of the intraepithelial lymphocyte populations associated with structurally developed Peyer's patches of foetal sheep and those of lambs allowed assessment of the impact of extrinsic factors from which the sheep foetus is shielded. The study confirmed the postnatal expansion in the villous intraepithelial lymphocyte population and showed that this expansion involved the CD8 and gamma delta phenotypes. CD4 lymphocytes did not appear in the follicle-associated epithelium until after birth. Unlike the villous epithelium, the follicle-associated epithelium had a high frequency of IgM+ and MHC II+ cells, which was dramatically reduced after birth. This postnatal reduction was particularly prominant in the follicle-associated epithelium of the jejunal Peyer's patch, where the frequency of IgM+ cells fell from 12.4% in foetal sheep to 0.7% in lambs. Double staining for alkaline phosphatase in the jejunal Peyer's patch suggested that clusters of IgM+ cells were associated with M cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beh K. J. Monoclonal antibodies against sheep immunoglobulin light chain, IgM and IgA. Vet Immunol Immunopathol. 1988 Feb;18(1):19–27. doi: 10.1016/0165-2427(88)90033-5. [DOI] [PubMed] [Google Scholar]
- Beya M. F., Miyasaka M., Dudler L., Ezaki T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. II. Two monoclonal antibodies that recognize all ovine T lymphocytes. Immunology. 1986 Jan;57(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- Bjerke K., Brandtzaeg P., Fausa O. T cell distribution is different in follicle-associated epithelium of human Peyer's patches and villous epithelium. Clin Exp Immunol. 1988 Nov;74(2):270–275. [PMC free article] [PubMed] [Google Scholar]
- Boyd R. D., Haworth C., Stacey T. E., Ward H. T. Permeability of the sheep placenta to unmetabolized polar non-electrolytes. J Physiol. 1976 Apr;256(3):617–634. doi: 10.1113/jphysiol.1976.sp011342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Sollid L. M., Thrane P. S., Kvale D., Bjerke K., Scott H., Kett K., Rognum T. O. Lymphoepithelial interactions in the mucosal immune system. Gut. 1988 Aug;29(8):1116–1130. doi: 10.1136/gut.29.8.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen H., Heremans J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970 May;22(5):448–457. [PubMed] [Google Scholar]
- Ermak T. H., Owen R. L. Differential distribution of lymphocytes and accessory cells in mouse Peyer's patches. Anat Rec. 1986 Jun;215(2):144–152. doi: 10.1002/ar.1092150208. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes in the small intestine of the mouse. Clin Exp Immunol. 1972 Dec;12(4):477–488. [PMC free article] [PubMed] [Google Scholar]
- Gogolin-Ewens K. J., Mackay C. R., Mercer W. R., Brandon M. R. Sheep lymphocyte antigens (OLA). I. Major histocompatibility complex class I molecules. Immunology. 1985 Dec;56(4):717–723. [PMC free article] [PubMed] [Google Scholar]
- Goodman T., Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989 Nov 1;170(5):1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleraker M., Landsverk T., Nicander L. Organization of ruminant Peyer's patches as seen with enzyme histochemical markers of stromal and accessory cells. Vet Immunol Immunopathol. 1990 Sep;26(1):93–104. doi: 10.1016/0165-2427(90)90135-f. [DOI] [PubMed] [Google Scholar]
- Hopkins J., Dutia B. M., McConnell I. Monoclonal antibodies to sheep lymphocytes. I. Identification of MHC class II molecules on lymphoid tissue and changes in the level of class II expression on lymph-borne cells following antigen stimulation in vivo. Immunology. 1986 Nov;59(3):433–438. [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr Frontiers of the immune system. Nature. 1988 Jun 30;333(6176):804–806. doi: 10.1038/333804a0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Jarry A., Robaszkiewicz M., Brousse N., Potet F. Immune cells associated with M cells in the follicle-associated epithelium of Peyer's patches in the rat. An electron- and immuno-electron-microscopic study. Cell Tissue Res. 1989 Feb;255(2):293–298. doi: 10.1007/BF00224111. [DOI] [PubMed] [Google Scholar]
- Landsverk T., Jansson A., Nicander L., Pløen L. Carbonic anhydrase--a marker for particles shed from the epithelium to the lymphoid follicles of the ileal Peyer's patch in goat kids and lambs. Immunol Cell Biol. 1987 Oct;65(Pt 5):425–429. doi: 10.1038/icb.1987.49. [DOI] [PubMed] [Google Scholar]
- Landsverk T. The follicle-associated epithelium of the ileal Peyer's patch in ruminants is distinguished by its shedding of 50 nm particles. Immunol Cell Biol. 1987 Jun;65(Pt 3):251–261. doi: 10.1038/icb.1987.28. [DOI] [PubMed] [Google Scholar]
- Landsverk T., Trevella W., Nicander L. Transfer of carbonic anhydrase-positive particles from the follicle-associated epithelium to lymphocytes of Peyer's patches in foetal sheep and lambs. Cell Tissue Res. 1990 Aug;261(2):239–247. doi: 10.1007/BF00318665. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Ferguson A. Small intestinal epithelial cell kinetics and protozoal infection in mice. Gastroenterology. 1978 Mar;74(3):496–500. [PubMed] [Google Scholar]
- Mackay C. R., Beya M. F., Matzinger P. Gamma/delta T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989 Aug;19(8):1477–1483. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Bhalla D. K. Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer's patch M cells. Am J Anat. 1983 Oct;168(2):199–212. doi: 10.1002/aja.1001680207. [DOI] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Reynolds J. D., Morris B. The influence of gut function on lymphoid cell populations in the intestinal mucosa of lambs. Immunology. 1983 Jul;49(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Reynolds J., Pabst R., Bordmann G. Evidence for the existence of two distinct types of Peyer's patches in sheep. Adv Exp Med Biol. 1985;186:101–109. doi: 10.1007/978-1-4613-2463-8_12. [DOI] [PubMed] [Google Scholar]
- Wilders M. M., Sminia T., Plesch B. E., Drexhage H. A., Weltevreden E. F., Meuwissen S. G. Large mononuclear Ia-positive veiled cells in Peyer's patches. II. Localization in rat Peyer's patches. Immunology. 1983 Mar;48(3):461–467. [PMC free article] [PubMed] [Google Scholar]