Abstract
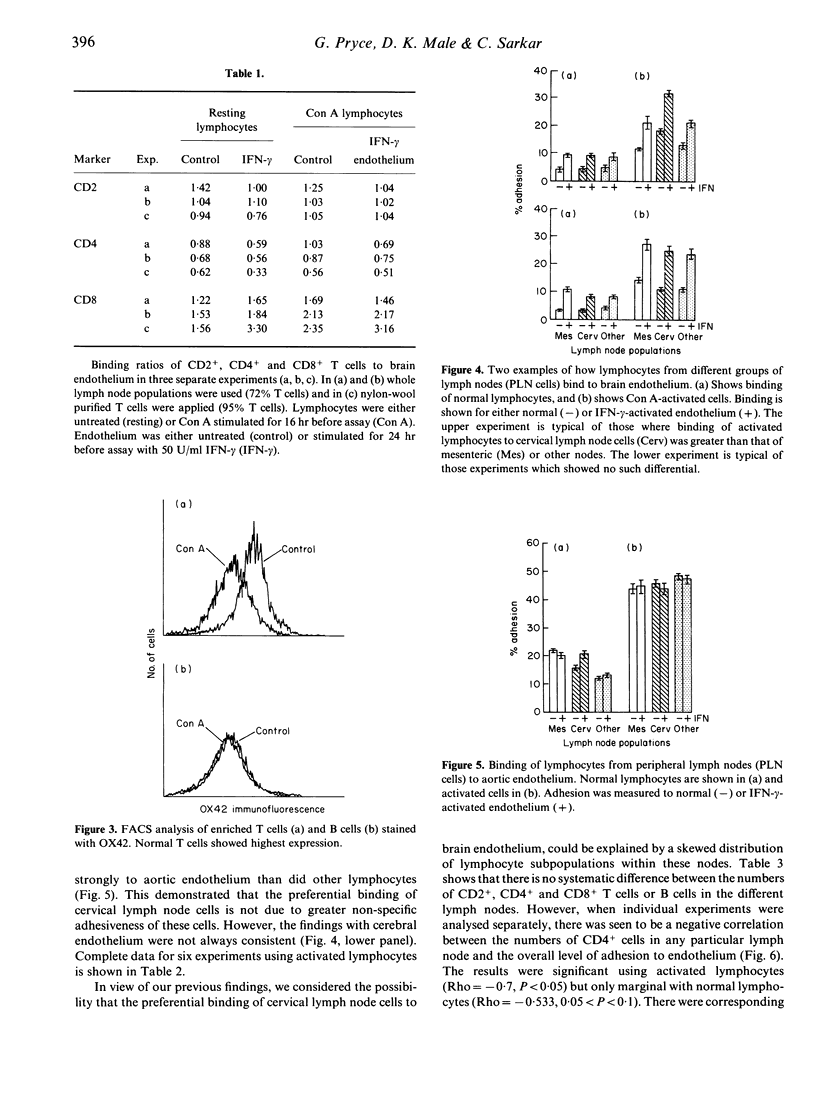
We have determined whether particular lymphocyte populations bind preferentially to cerebral endothelium, using adhesion assays and a new method for in situ staining of adherent lymphocytes. B cells bind more strongly than T cells, an effect enhanced by lymphocyte activation or endothelial cell stimulation with interferon-gamma (IFN-gamma) or tumour necrosis factor-alpha (TNF-alpha). This is not equated with levels of CD18 expression on the lymphocytes. CD8+ T cells bound more efficiently than CD4+ cells under all conditions. To determine whether there was a population of cells which selectively homes to the brain, we compared adhesion of cervical lymph nodes cells to brain endothelium, with adhesion of lymphocytes from other nodes. In 50% of the experiments there was significantly enhanced binding of activated cervical lymph cells to cerebral endothelium but not to control (aortic) endothelium. This effect was seen using both normal and IFN-gamma-activated endothelium. The explanation for this finding is that cervical lymph nodes frequently, but not invariably, contain higher proportions of CD8+ cells and B cells than other lymph nodes. These data imply that selective adhesion of lymphocytes to brain endothelium is related to the subpopulations involved and this may be reflected in the cell types seen in immunological lesions of the brain, and in the relative proportions of the subpopulations seen in cervical lymph nodes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Alby L., Morrissey L. W., Tu M., Joseph J. Expression of organ-specific antigens on capillary endothelial cells. Microvasc Res. 1985 May;29(3):401–411. doi: 10.1016/0026-2862(85)90028-7. [DOI] [PubMed] [Google Scholar]
- Bender J. R., Pardi R., Karasek M. A., Engleman E. G. Phenotypic and functional characterization of lymphocytes that bind human microvascular endothelial cells in vitro. Evidence for preferential binding of natural killer cells. J Clin Invest. 1987 Jun;79(6):1679–1688. doi: 10.1172/JCI113007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G. W., Betz A. L., Bowman P. D. Use of isolated brain capillaries and cultured endothelial cells to study the blood-brain barrier. Fed Proc. 1984 Feb;43(2):191–195. [PubMed] [Google Scholar]
- Hafler D. A., Weiner H. L. T cells in multiple sclerosis and inflammatory central nervous system diseases. Immunol Rev. 1987 Dec;100:307–332. doi: 10.1111/j.1600-065x.1987.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Harling-Berg C., Knopf P. M., Merriam J., Cserr H. F. Role of cervical lymph nodes in the systemic humoral immune response to human serum albumin microinfused into rat cerebrospinal fluid. J Neuroimmunol. 1989 Dec;25(2-3):185–193. doi: 10.1016/0165-5728(89)90136-7. [DOI] [PubMed] [Google Scholar]
- Hauser S. L., Bhan A. K., Gilles F., Kemp M., Kerr C., Weiner H. L. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986 Jun;19(6):578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Gonatas N. K. Suppressor T-lymphocytes in the spinal cord of Lewis rats recovered from acute experimental allergic encephalomyelitis. Cell Immunol. 1984 Apr 15;85(1):284–288. doi: 10.1016/0008-8749(84)90300-9. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Lantos P. L. Brain capillary endothelial cells in vitro lack surface IgG Fc receptors. Neurosci Lett. 1986 Jul 11;68(1):100–106. doi: 10.1016/0304-3940(86)90237-5. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Male D. K., Lantos P. L. Adhesion of lymphocytes to cerebral microvascular cells: effects of interferon-gamma, tumour necrosis factor and interleukin-1. Immunology. 1988 Aug;64(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., Webster D. M. Role of interferon in lymphocyte recruitment into the skin. Cell Immunol. 1986 May;99(2):322–333. doi: 10.1016/0008-8749(86)90241-8. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Male D. K., Pryce G., Hughes C. C. Antigen presentation in brain: MHC induction on brain endothelium and astrocytes compared. Immunology. 1987 Mar;60(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Male D., Pryce G., Rahman J. Comparison of the immunological properties of rat cerebral and aortic endothelium. J Neuroimmunol. 1990 Dec;30(2-3):161–168. doi: 10.1016/0165-5728(90)90100-2. [DOI] [PubMed] [Google Scholar]
- Male D., Pyrce G., Hughes C., Lantos P. Lymphocyte migration into brain modelled in vitro: control by lymphocyte activation, cytokines, and antigen. Cell Immunol. 1990 Apr 15;127(1):1–11. doi: 10.1016/0008-8749(90)90109-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. Adoptively transferred experimental allergic encephalomyelitis in chimeric rats: identification of transferred cells in the lesions of the central nervous system. Immunology. 1988 Sep;65(1):23–29. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Hara N., Tanaka R., Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986 May 15;136(10):3668–3676. [PubMed] [Google Scholar]
- McCallum K., Esiri M. M., Tourtellotte W. W., Booss J. T cell subsets in multiple sclerosis. Gradients at plaque borders and differences in nonplaque regions. Brain. 1987 Oct;110(Pt 5):1297–1308. doi: 10.1093/brain/110.5.1297. [DOI] [PubMed] [Google Scholar]
- Picciano P. T., Johnson B., Walenga R. W., Donovan M., Borman B. J., Douglas W. H., Kreutzer D. L. Effects of D-valine on pulmonary artery endothelial cell morphology and function in cell culture. Exp Cell Res. 1984 Mar;151(1):134–147. doi: 10.1016/0014-4827(84)90363-x. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Sedgwick J. D., Mason D. W. The mechanism of inhibition of experimental allergic encephalomyelitis in the rat by monoclonal antibody against CD4. J Neuroimmunol. 1986 Dec;13(2):217–232. doi: 10.1016/0165-5728(86)90066-4. [DOI] [PubMed] [Google Scholar]
- Traugott U., Reinherz E. L., Raine C. S. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983 Jan 21;219(4582):308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., van de Wiel-van Kemenade P., Weder P., Kuijpers T. W., Figdor C. G. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989 Dec 14;342(6251):811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]