Abstract
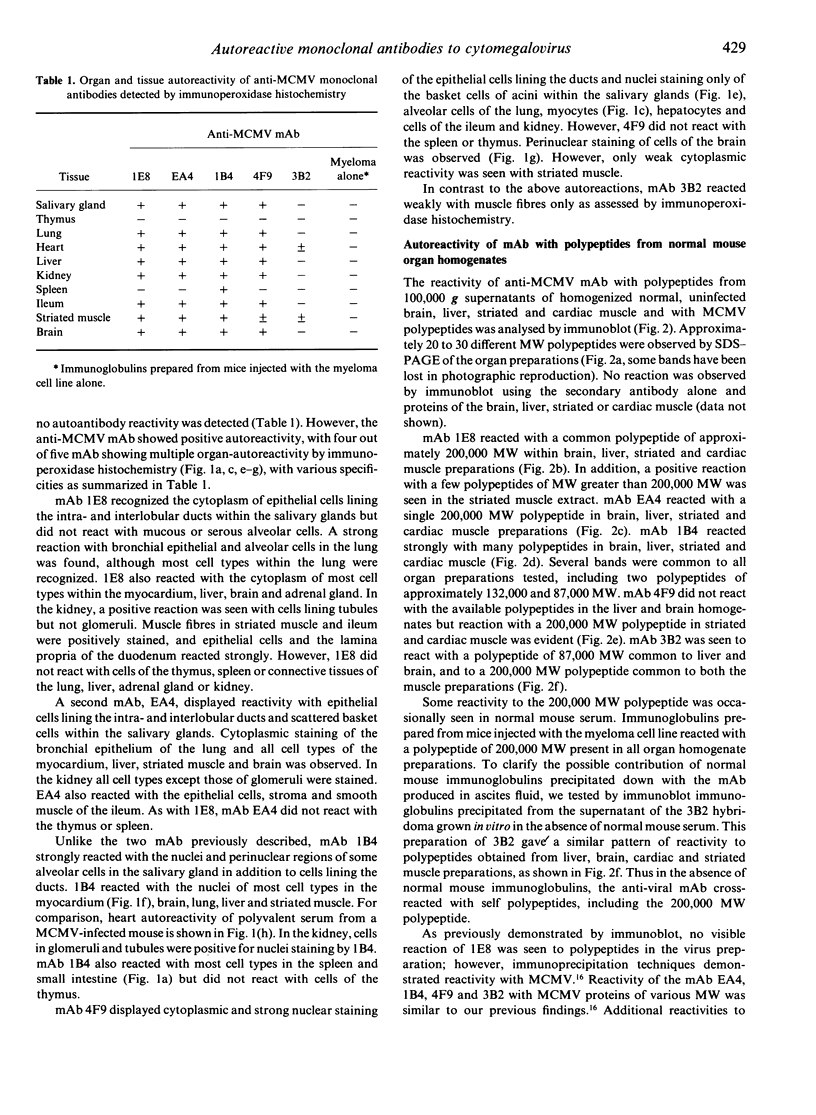
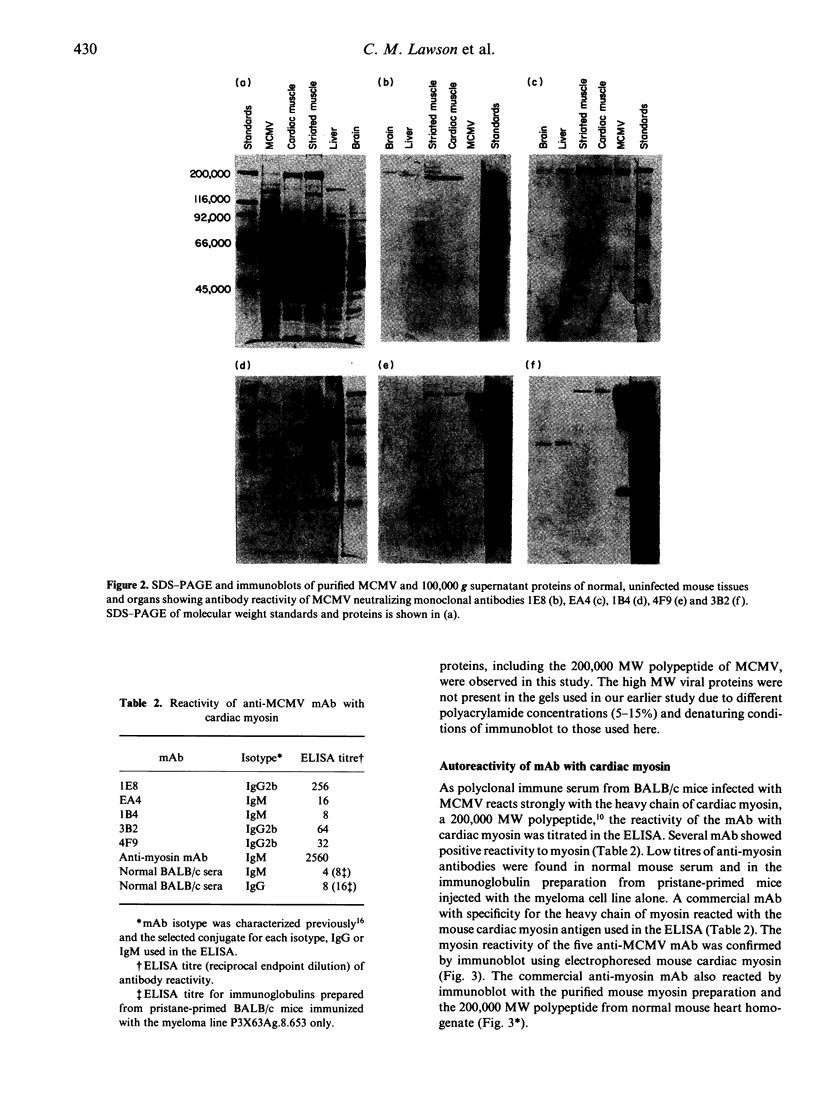
Certain murine monoclonal antibodies (mAb) raised against structural proteins of mouse cytomegalovirus (MCMV) display distinct patterns of multiple organ-autoreactivity in addition to their viral specificities. We analysed the autoreactivity of five such mAb by immunoperoxidase histochemistry, western immunoblot and enzyme-linked immunosorbent assay (ELISA). Four mAb recognized cellular autoantigens in the salivary gland, lung, heart, liver, kidney, ileum, striated muscle and brain, as detected by immunoperoxidase histochemistry. However, the mAb showed different specificities for nuclear, cytoplasmic and surface membrane antigens on various cell types in addition to common autoreactivities. Immunoblot analyses showed that some of the mAb recognized polypeptides of various molecular weights obtained from 100,000 g supernatants of normal BALB/c liver, brain, striated and cardiac muscle homogenates. Reactivity of the mAb with a 200,000 molecular weight (MW) polypeptide was similar to our previous finding of the reaction of late immune polyclonal sera with a 200,000 MW polypeptide, the heavy chain of myosin. The mAb reacted to the cardiac isoform of myosin as determined by ELISA and immunoblot. Reactivity of mAb with cardiac myosin, as detected by immunoblot, was removed by absorption with cardiac myosin and recovered in the eluate. However, cardiac myosin used in a competitive inhibition ELISA did not abrogate the reactivity of the mAb with MCMV antigens. These anti-MCMV mAb appear to be multispecific for both virus and self-antigens, including cardiac myosin, and possibly recognize these different antigens through partly similar or distinct antigen-binding sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Andersen H. K. Smooth-muscle antibodies and other tissue antibodies in cytomegalovirus infection. Clin Exp Immunol. 1975 Oct;22(1):22–29. [PMC free article] [PubMed] [Google Scholar]
- Araullo-Cruz T. P., Ho M., Armstrong J. A. Protective effect of early serum from mice after cytomegalovirus infection. Infect Immun. 1978 Sep;21(3):840–842. doi: 10.1128/iai.21.3.840-842.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomaeus W. N., O'Donoghue H., Foti D., Lawson C. M., Shellam G. R., Reed W. D. Multiple autoantibodies following cytomegalovirus infection: virus distribution and specificity of autoantibodies. Immunology. 1988 Jul;64(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Beck S., Barrell B. G. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988 Jan 21;331(6153):269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- Chee M. S., Satchwell S. C., Preddie E., Weston K. M., Barrell B. G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990 Apr 19;344(6268):774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Mazié J. C., Rouyre S., Butler-Browne G. S., Whalen R. G., Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983 Nov;131(5):2267–2272. [PubMed] [Google Scholar]
- Farrell H. E., Shellam G. R. Characterization of neutralizing monoclonal antibodies to murine cytomegalovirus. J Gen Virol. 1990 Mar;71(Pt 3):655–664. doi: 10.1099/0022-1317-71-3-655. [DOI] [PubMed] [Google Scholar]
- Farrell H. E., Shellam G. R. Immunoblot analysis of the antibody response to murine cytomegalovirus in genetically resistant and susceptible mice. J Gen Virol. 1989 Oct;70(Pt 10):2573–2586. doi: 10.1099/0022-1317-70-10-2573. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Nelson J. A., Walker L., Oldstone M. B. Sequence homology and immunologic cross-reactivity of human cytomegalovirus with HLA-DR beta chain: a means for graft rejection and immunosuppression. J Virol. 1988 Jan;62(1):100–105. doi: 10.1128/jvi.62.1.100-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., McClintock P. R., Essani K., Ray U. R., Yagihashi S., Notkins A. L. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983 Jul 7;304(5921):73–76. doi: 10.1038/304073a0. [DOI] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., Elkins M. H. B cell activation by cytomegalovirus. J Exp Med. 1983 Dec 1;158(6):2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Lawson C. M., Grundy J. E., Shellam G. R. Antibody responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1988 Aug;69(Pt 8):1987–1998. doi: 10.1099/0022-1317-69-8-1987. [DOI] [PubMed] [Google Scholar]
- Luka J., Kreofsky T., Pearson G. R., Hennessy K., Kieff E. Identification and characterization of a cellular protein that cross-reacts with the Epstein-Barr virus nuclear antigen. J Virol. 1984 Dec;52(3):833–838. doi: 10.1128/jvi.52.3.833-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson S., Tardy-Panit M., Colimon R., Landini M. P. A human cytomegalovirus-neutralizing monoclonal antibody recognizes a normal cell protein. J Gen Virol. 1989 Mar;70(Pt 3):673–684. doi: 10.1099/0022-1317-70-3-673. [DOI] [PubMed] [Google Scholar]
- O'Donoghue H. L., Lawson C. M., Reed W. D. Autoantibodies to cardiac myosin in mouse cytomegalovirus myocarditis. Immunology. 1990 Sep;71(1):20–28. [PMC free article] [PubMed] [Google Scholar]
- Onodera T., Ray U. R., Melez K. A., Suzuki H., Toniolo A., Notkins A. L. Virus-induced diabetes mellitus: autoimmunity and polyendocrine disease prevented by immunosuppression. Nature. 1982 May 6;297(5861):66–68. doi: 10.1038/297066a0. [DOI] [PubMed] [Google Scholar]
- Saegusa J., Prabhakar B. S., Essani K., McClintock P. R., Fukuda Y., Ferrans V. J., Notkins A. L. Monoclonal antibody to coxsackievirus B4 reacts with myocardium. J Infect Dis. 1986 Feb;153(2):372–373. doi: 10.1093/infdis/153.2.372. [DOI] [PubMed] [Google Scholar]
- Shillitoe E. J., Daniels T. E., Whitcher J. P., Vibeke Strand C., Talal N., Greenspan J. S. Antibody to cytomegalovirus in patients with Sjögren's syndrome. As determined by an enzyme-linked immunosorbent assay. Arthritis Rheum. 1982 Mar;25(3):260–265. doi: 10.1002/art.1780250303. [DOI] [PubMed] [Google Scholar]
- Srinivasappa J., Saegusa J., Prabhakar B. S., Gentry M. K., Buchmeier M. J., Wiktor T. J., Koprowski H., Oldstone M. B., Notkins A. L. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986 Jan;57(1):397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S. I., Spector D. H. 2.2-kilobase class of early transcripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J Virol. 1986 Feb;57(2):591–602. doi: 10.1128/jvi.57.2.591-602.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager O., Räsänen J. A., Hagman A., Klemola E. Mixed cryoimmunoglobulinaemia in infectious mononucleois and Cytomegalovirus mononucleosis. Int Arch Allergy Appl Immunol. 1968;34(4):345–361. doi: 10.1159/000230129. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Hauser S. L. Neuroimmunology I: Immunoregulation in neurological disease. Ann Neurol. 1982 May;11(5):437–449. doi: 10.1002/ana.410110502. [DOI] [PubMed] [Google Scholar]