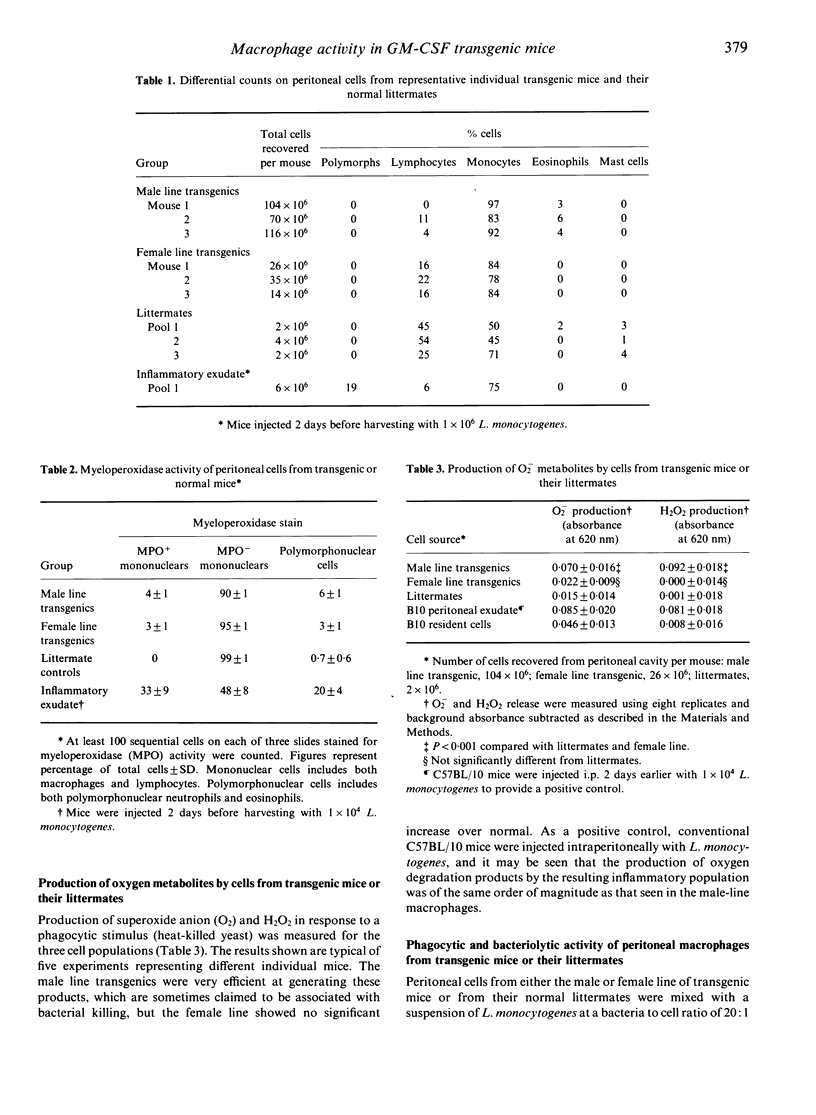
Abstract
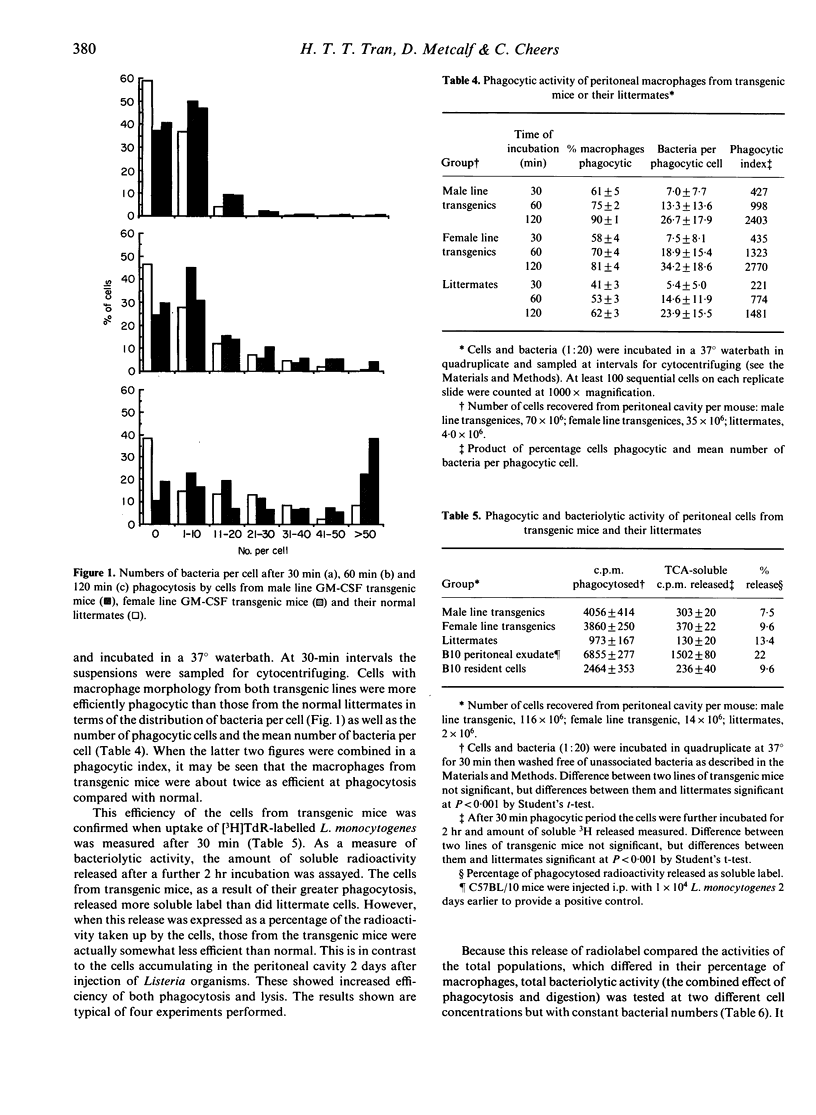
Two lines of transgenic mice carrying the gene for granulocyte-macrophage colony-stimulating factor (GM-CSF) produce vastly increased numbers of macrophages with abundant foamy cytoplasm resembling classical activated macrophages. Cells from both lines were negative for myeloperoxidase, a bactericidal enzyme found in monocytes as well as neutrophils, but not mature macrophages. Cells from the so called 'male line' produced greatly increased levels of oxygen degradation products in response to phagocytosis, compared with cells from the 'female line' or from normal littermates. The ability of the cells to phagocytose and lyse the intracellular bacterium Listeria monocytogenes was tested in vitro using radiolabelled organisms. Although the cells from transgenic mice were more highly phagocytic than cells from normal littermates, cells from either line were no more efficient than normal at lysing the bacteria they had phagocytosed. Nevertheless, because of the high phagocytic rate, more bacteria were exposed to lysis in the cells of transgenic mice, and the final outcome was a higher rate of bacteriolysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Bortolussi R., Vandenbroucke-Grauls C. M., van Asbeck B. S., Verhoef J. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987 Dec;55(12):3197–3203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Haigh A. M., Kelso A., Metcalf D., Stanley E. R., Young A. M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988 Jan;56(1):247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Hill M., Haigh A. M., Stanley E. R. Stimulation of macrophage phagocytic but not bactericidal activity by colony-stimulating factor 1. Infect Immun. 1989 May;57(5):1512–1516. doi: 10.1128/iai.57.5.1512-1516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Stanley E. R. Macrophage production during murine listeriosis: colony-stimulating factor 1 (CSF-1) and CSF-1-binding cells in genetically resistant and susceptible mice. Infect Immun. 1988 Nov;56(11):2972–2978. doi: 10.1128/iai.56.11.2972-2978.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W. A. Kinetics of killing Listeria monocytogenes by macrophages: rapid killing accompanying phagocytosis. J Reticuloendothel Soc. 1983 Aug;34(2):131–141. [PubMed] [Google Scholar]
- Gearing A. J., Metcalf D., Moore J. G., Nicola N. A. Elevated levels of GM-CSF and IL-1 in the serum, peritoneal and pleural cavities of GM-CSF transgenic mice. Immunology. 1989 Jun;67(2):216–220. [PMC free article] [PubMed] [Google Scholar]
- Handman E., Burgess A. W. Stimulation by granulocyte-macrophage colony-stimulating factor of Leishmania tropica killing by macrophages. J Immunol. 1979 Mar;122(3):1134–1137. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Karbassi A., Becker J. M., Foster J. S., Moore R. N. Enhanced killing of Candida albicans by murine macrophages treated with macrophage colony-stimulating factor: evidence for augmented expression of mannose receptors. J Immunol. 1987 Jul 15;139(2):417–421. [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Lee M. T., Warren M. K. CSF-1-induced resistance to viral infection in murine macrophages. J Immunol. 1987 May 1;138(9):3019–3022. [PubMed] [Google Scholar]
- Metcalf D., Moore J. G. Divergent disease patterns in granulocyte-macrophage colony-stimulating factor transgenic mice associated with different transgene insertion sites. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7767–7771. doi: 10.1073/pnas.85.20.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Quesenberry P., Cohen H., Levin J., Sullivan R., Bealmear P., Ryan M. Effects of bacterial infection and irradiation on serum colony-stimulating factor levels in tolerant and nontolerant CF1 mice. Blood. 1978 Feb;51(2):229–244. [PubMed] [Google Scholar]
- Rook G. A., Steele J., Umar S., Dockrell H. M. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods. 1985 Sep 3;82(1):161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- Trudgett A., McNeill T. A., Killen M. Granulocyte-macrophage precursor cell and colony-stimulating factor responses of mice infected with Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):450–455. doi: 10.1128/iai.8.3.450-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. R., Spanidis V., Frangos K., Cheers C. The in vitro bactericidal activity of peritoneal and spleen cells from Listeria-resistant and -susceptible mouse strains. Cell Immunol. 1986 Apr 15;99(1):160–169. doi: 10.1016/0008-8749(86)90225-x. [DOI] [PubMed] [Google Scholar]
- Young A. M., Cheers C. Colony-forming cells and colony-stimulating activity during listeriosis in genetically resistant or susceptible mice. Cell Immunol. 1986 Feb;97(2):227–237. doi: 10.1016/0008-8749(86)90393-x. [DOI] [PubMed] [Google Scholar]


