Abstract
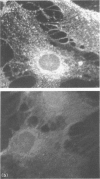
We have studied the adhesion of human CD4+ lymphocytes to cultured human retinal vascular endothelial cells (EC) and human retinal pigment epithelial cells (RPE), both of which comprise the cellular components of the blood-retina barrier. We have observed differences in the lymphocyte-RPE and the lymphocyte-EC interactions. Firstly, RPE cells were found to express high levels of the adhesion molecule ICAM-1 constitutively, whereas EC expressed ICAM-1 only after induction with IFN-gamma. In addition, lymphocyte binding to normal and minimally stimulated RPE (5 U/ml, 4 hr) was predominantly ICAM-1 dependent, but after maximal stimulation (500 U/ml, 4 days), increased lymphocyte adhesiveness included an ICAM-1-independent component, which was apparently not due to involvement of MHC class II or CD2 molecules. In contrast, binding of lymphocytes to unstimulated EC involved both an ICAM-1-dependent and an ICAM-1-independent mechanism, the latter being subject to inhibition by monoclonal antibody to CD2. Studies of adhesion at 4 indicated that no binding occurred to normal or stimulated RPE, but binding to EC was observed, albeit reduced to 50% of the 37 binding level, and this implies that the LFA-3/CD2 adhesion pathway may also be involved in lymphocyte binding to EC. Overall, the results indicate a functional difference between RPE and EC affecting T-cell adhesion, migration and activation at the blood-retinal barrier, which must be considered when devising therapies to prevent lymphocyte infiltration of the eye.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Yossefi S. Reversal of autoimmune encephalomyelitis by membranes presenting myelin basic protein-associated class II MHC molecule as an approach to immunotherapy of organ-specific autoimmune diseases. Eur J Immunol. 1990 Feb;20(2):357–361. doi: 10.1002/eji.1830200219. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Sjögren H. O., Carlsson R. Two subsets of human CD4+ T helper cells differing in kinetics and capacities to produce interleukin 2 and interferon-gamma can be defined by the Leu-18 and UCHL1 monoclonal antibodies. Eur J Immunol. 1988 Aug;18(8):1173–1178. doi: 10.1002/eji.1830180805. [DOI] [PubMed] [Google Scholar]
- Donoso L. A., Merryman C. F., Sery T., Sanders R., Vrabec T., Fong S. L. Human interstitial retinoid binding protein. A potent uveitopathogenic agent for the induction of experimental autoimmune uveitis. J Immunol. 1989 Jul 1;143(1):79–83. [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., Borthwick G. M., McMenamin P. G. Ultrastructural pathology of S-antigen uveoretinitis. Invest Ophthalmol Vis Sci. 1985 Sep;26(9):1281–1292. [PubMed] [Google Scholar]
- Friedrich B., Cantrell D. A., Gullberg M. Evidences for protein kinase C. Activation in T lymphocytes by stimulation of either the CD2 or CD3 antigens. Eur J Immunol. 1989 Jan;19(1):17–23. doi: 10.1002/eji.1830190104. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Fling S. P., Obritsch W. F., Merryman C. F., Donoso L. A. Identification of T cell recognition sites in S-antigen: dissociation of proliferative and pathogenic sites. Cell Immunol. 1989 Oct 15;123(2):427–440. doi: 10.1016/0008-8749(89)90302-x. [DOI] [PubMed] [Google Scholar]
- Liversidge J. M., Sewell H. F., Forrester J. V. Human retinal pigment epithelial cells differentially express MHC class II (HLA, DP, DR and DQ) antigens in response to in vitro stimulation with lymphokine or purified IFN-gamma. Clin Exp Immunol. 1988 Sep;73(3):489–494. [PMC free article] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Gugel E. A., Dustin M. L., Springer T. A., Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988 Apr;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- McIntosh L. C., Muckersie L., Forrester J. V. Retinal capillary endothelial cells prefer different substrates for growth and migration. Tissue Cell. 1988;20(2):193–209. doi: 10.1016/0040-8166(88)90041-9. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Chang H. C., Wallner B. P., Stebbins C., Frey A. Z., Reinherz E. L. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature. 1989 May 25;339(6222):312–314. doi: 10.1038/339312a0. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Sewell H. F., Milton J. I., MacKenzie R., King D. J., Dawson A. A., Lessels S. E., Bennett B., Davidson R. L., Walker F. Immunophenotyping of leukaemias by flow cytometry and APAAP: a two-year comparative analysis in hospital practice. Dis Markers. 1988 Oct-Dec;6(4):221–229. [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., van de Wiel-van Kemenade P., Weder P., Kuijpers T. W., Figdor C. G. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989 Dec 14;342(6251):811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]