Abstract
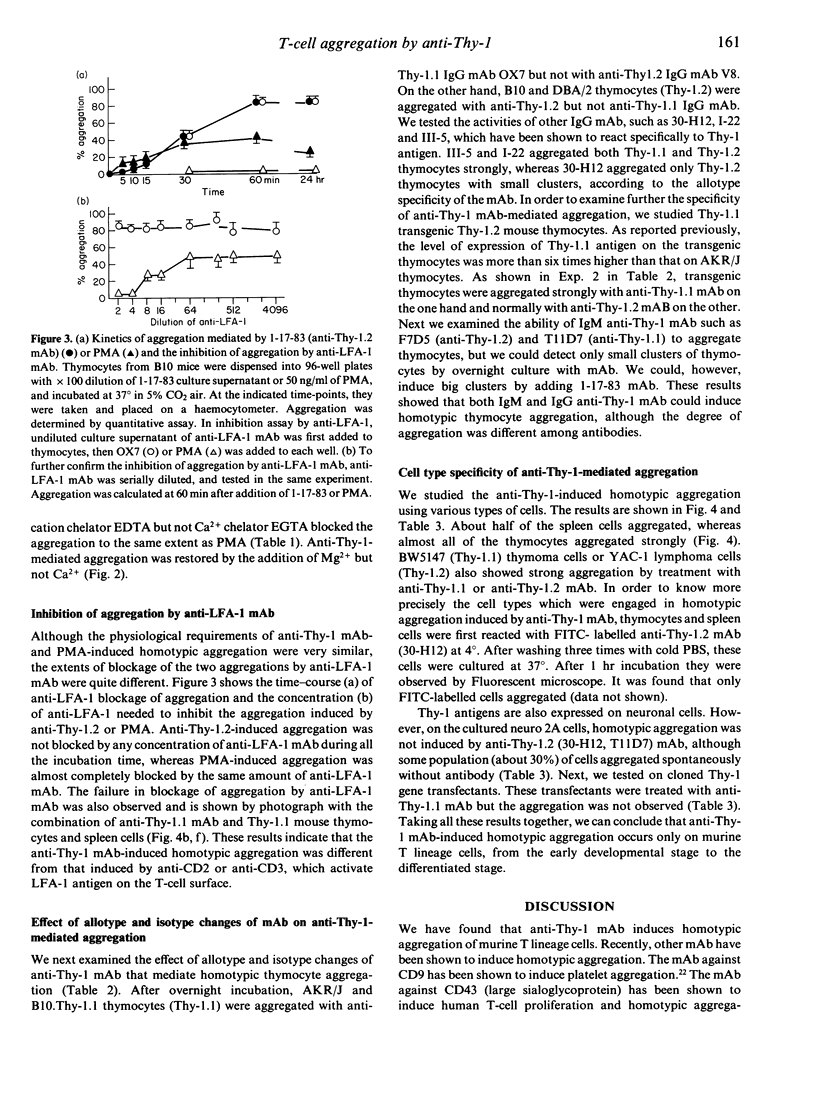
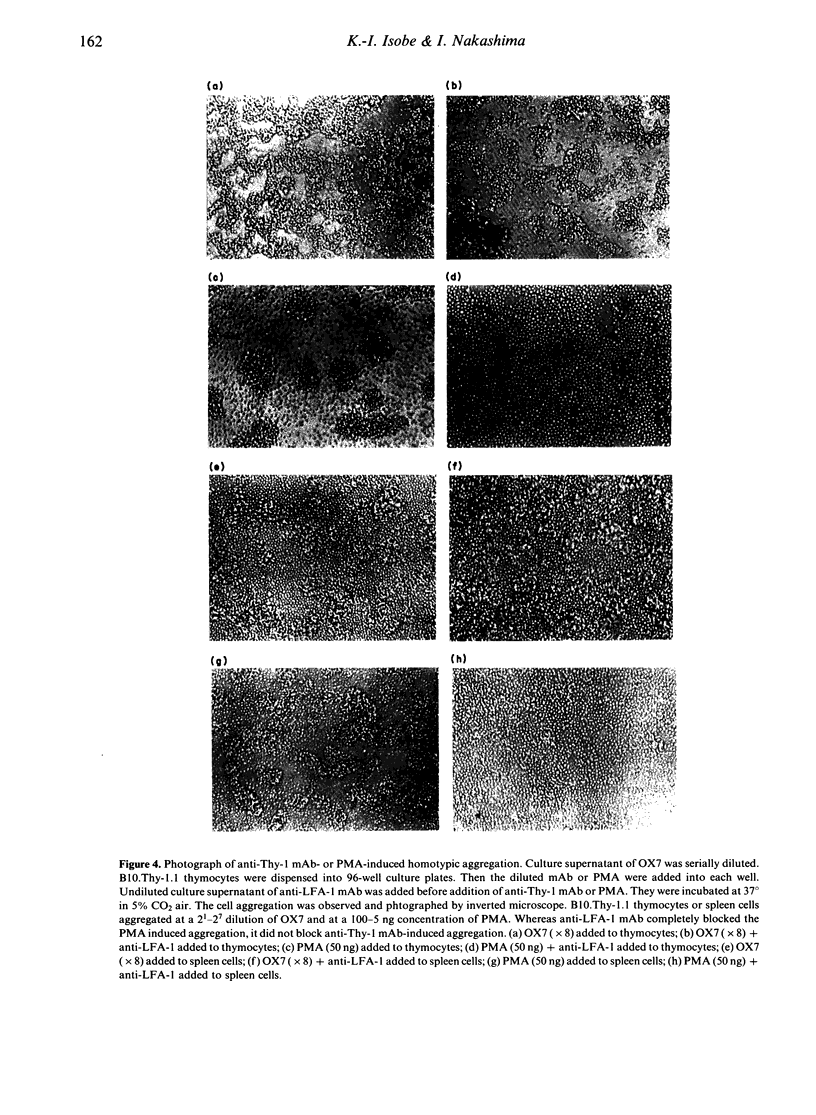
During the course of studies of anti-Thy-1-mediated T-cell activation, we found that anti-Thy-1 monoclonal antibodies (mAb) could induce strong homotypic aggregation of murine T-lineage cells. We demonstrated that anti-Thy-1 mAb-mediated T-cell aggregation started at 10 min and reached maximum level 1 hr after addition of antibody. It was temperature dependent, requiring metabolic energy and cytoskeletal integrity similar to that mediated by phorbol myristate acetate (PMA). But the striking difference between anti-Thy-1 mAb-mediated cell aggregation and PMA-mediated cell aggregation was that the latter but not the former was blocked by anti-LFA-1 mAb. This indicates that, unlike treatment with PMA, anti-CD2 mAb or anti-CD3 mAb, anti-Thy-1 mAb treatment of T lymphocytes does not induce LFA-1 activation for cell adhesion. Murine neuroblastoma cells were not induced to aggregate by anti-Thy-1 mAb treatment, although murine T lymphoma cells were aggregated by anti-Thy-1 mAb. The T-lineage cell specificity of anti-Thy-1-mediated aggregation was further shown by the Thy-1 gene transfection into non-Thy-1 expressing cell lines. Thy-1.1 gene transfected mastocytoma cells were not aggregated by anti-Thy-1.1 antibody.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson B., Youseffi-Etemad R., Hammarström S., Perlmann P. Induction of aggregation and enhancement of proliferation and IL-2 secretion in human T cells by antibodies to CD43. J Immunol. 1988 Nov 1;141(9):2912–2917. [PubMed] [Google Scholar]
- Bednarczyk J. L., McIntyre B. W. A monoclonal antibody to VLA-4 alpha-chain (CDw49d) induces homotypic lymphocyte aggregation. J Immunol. 1990 Feb 1;144(3):777–784. [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase-bound OKT3. J Immunol. 1986 Sep 15;137(6):1816–1821. [PubMed] [Google Scholar]
- Fleischer B. A novel pathway of human T cell activation via a 103 kD T cell activation antigen. J Immunol. 1987 Mar 1;138(5):1346–1350. [PubMed] [Google Scholar]
- Giguére V., Isobe K., Grosveld F. Structure of the murine Thy-1 gene. EMBO J. 1985 Aug;4(8):2017–2024. doi: 10.1002/j.1460-2075.1985.tb03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter K. C., Germain R. N., Kroczek R. A., Saito T., Yokoyama W. M., Chan C., Weiss A., Shevach E. M. Thy-1-mediated T-cell activation requires co-expression of CD3/Ti complex. Nature. 1987 Apr 2;326(6112):505–507. doi: 10.1038/326505a0. [DOI] [PubMed] [Google Scholar]
- Gunter K. C., Malek T. R., Shevach E. M. T cell-activating properties of an anti-Thy-1 monoclonal antibody. Possible analogy to OKT3/Leu-4. J Exp Med. 1984 Mar 1;159(3):716–730. doi: 10.1084/jem.159.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashihara M., Maeda H., Shibata Y., Kume S., Ohashi T. A monoclonal anti-human platelet antibody: a new platelet aggregating substance. Blood. 1985 Feb;65(2):382–391. [PubMed] [Google Scholar]
- Isobe K., Fortunato A., Giguere V., Grosveld F., Mitchison N. A. Anti-Thy-1 antibody responses evoked by Thy-1 antigen expressed in transfected mouse mastocytoma cells and rat fibroblast. Immunology. 1985 Nov;56(3):505–512. [PMC free article] [PubMed] [Google Scholar]
- Isobe K., Kollias G., Kolsto A. B., Grosveld F. T cell independent Thy-1 allo-antibody response with the use of transgenic mice. J Immunol. 1986 Oct 1;137(7):2089–2092. [PubMed] [Google Scholar]
- Keizer G. D., Visser W., Vliem M., Figdor C. G. A monoclonal antibody (NKI-L16) directed against a unique epitope on the alpha-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J Immunol. 1988 Mar 1;140(5):1393–1400. [PubMed] [Google Scholar]
- Kroczek R. A., Gunter K. C., Germain R. N., Shevach E. M. Thy-1 functions as a signal transduction molecule in T lymphocytes and transfected B lymphocytes. Nature. 1986 Jul 10;322(6075):181–184. doi: 10.1038/322181a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Bron C., Rousseaux M., Horvath C., Cerottini J. C. Production and characterization of monoclonal anti-Thy-1 antibodies that stimulate lymphokine production by cytolytic T cell clones. Eur J Immunol. 1985 May;15(5):495–501. doi: 10.1002/eji.1830150514. [DOI] [PubMed] [Google Scholar]
- Maino V. C., Norcross M. A., Perkins M. S., Smith R. T. Mechanism of Thy-1-mediated T cell activation: roles of Fc receptors, T200, Ia, and H-2 glycoproteins in accessory cell function. J Immunol. 1981 May;126(5):1829–1836. [PubMed] [Google Scholar]
- Malek T. R., Ortega G., Chan C., Kroczek R. A., Shevach E. M. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986 Sep 1;164(3):709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Muto M., Sado T., Hayata I., Nagasawa F., Kamisaku H., Kubo E. Reconfirmation of indirect induction of radiogenic lymphomas using thymectomized, irradiated B10 mice grafted with neonatal thymuses from Thy 1 congenic donors. Cancer Res. 1983 Aug;43(8):3822–3827. [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Olive D., Poggi A., Pozzan T., Moretta L., Moretta A. Antibody-induced modulation of the CD3/T cell receptor complex causes T cell refractoriness by inhibiting the early metabolic steps involved in T cell activation. J Exp Med. 1987 Aug 1;166(2):619–624. doi: 10.1084/jem.166.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Verhulst A. M., Guimezanes A., Boyer C., Poenie M., Tsien R., Buferne M., Hua C., Leserman L. Pleiotropic loss of activation pathways in a T-cell receptor alpha-chain deletion variant of a cytolytic T-cell clone. Nature. 1987 Feb 12;325(6105):628–631. doi: 10.1038/325628a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Van Pel A., De Plaen E., Boon T. Selection of highly transfectable variant from mouse mastocytoma P815. Somat Cell Mol Genet. 1985 Sep;11(5):467–475. doi: 10.1007/BF01534840. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y., van de Wiel-van Kemenade P., Weder P., Kuijpers T. W., Figdor C. G. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989 Dec 14;342(6251):811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]



