Abstract
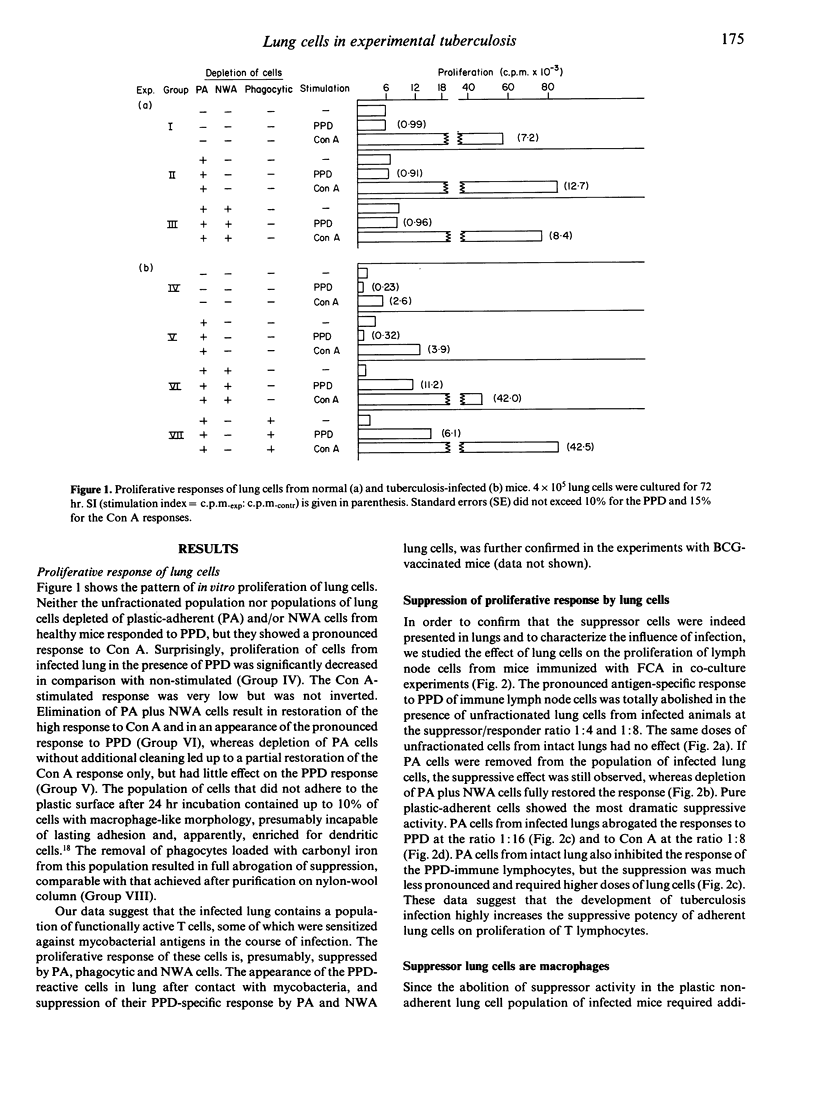
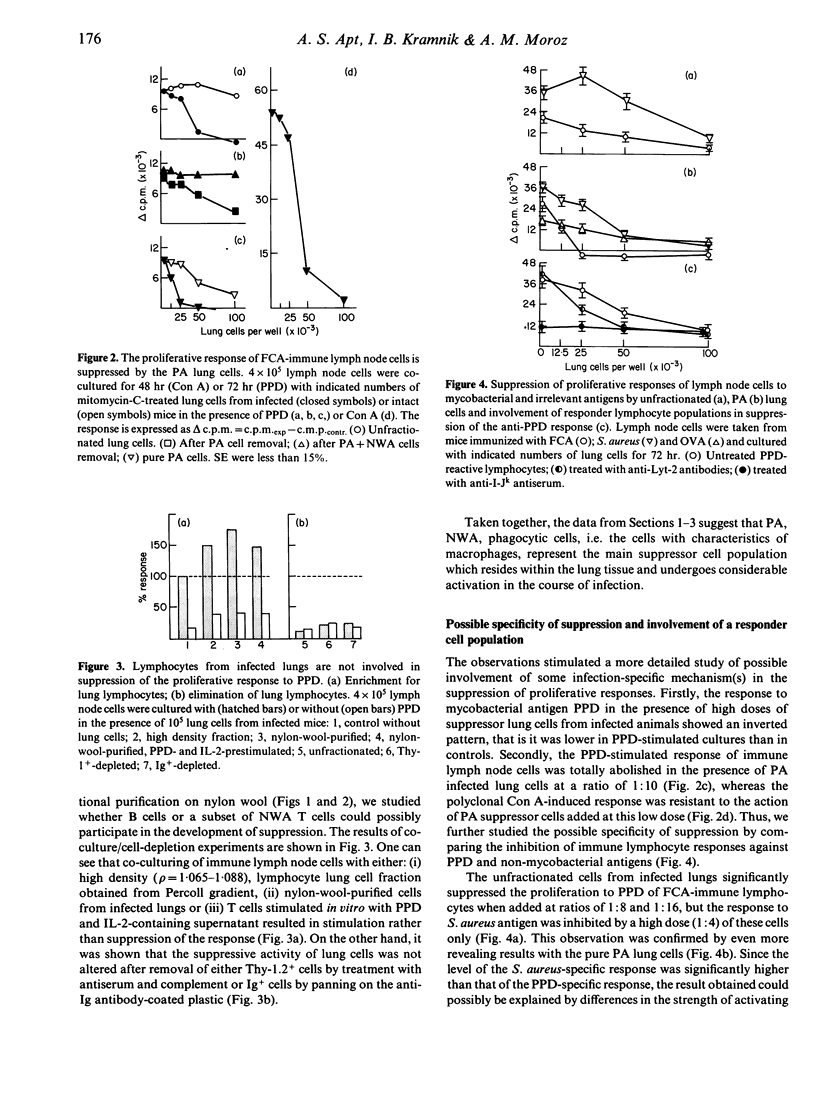
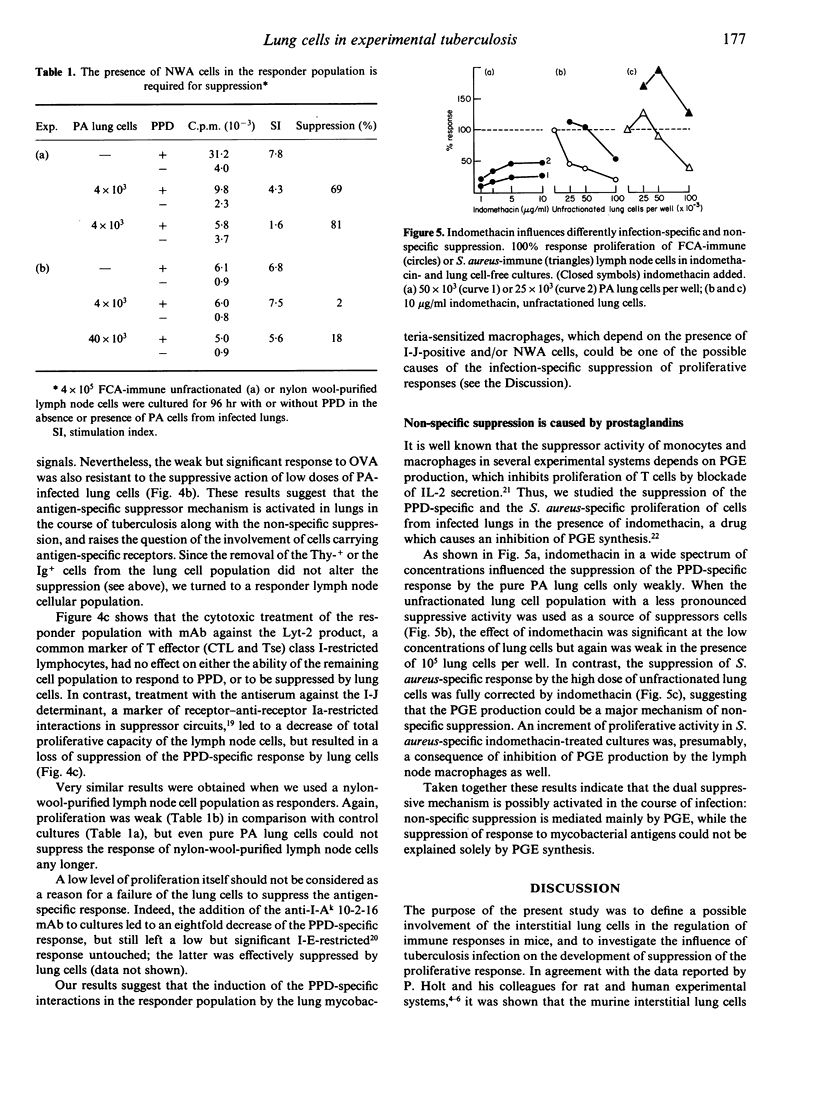
We have studied proliferative responses to mycobacterial antigen preparation (PPD) and to non-specific stimuli of interstitial cells from the lungs of Mycobacterium tuberculosis-infected CBA mice. PPD-reactive lymphocytes appeared in the lung wall tissue in the course of chronic infection, but their proliferative capacity was totally inhibited by the lung macrophages. The latter were also able to suppress the proliferation of immune lymph node T cells. The mechanism of suppression clearly had two components, one being infection-specific and the other non-specific. Non-specific suppression was mediated mainly by prostaglandin E(PGE), whereas the specific mechanism showed only a weak influence of PGE and depended on the presence of I-J+ Lyt-2- nylon-wool-adherent cells in the responder population. Interstitial lung T or B lymphocytes were not involved in specific suppression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilyk N., Mackenzie J. S., Papadimitriou J. M., Holt P. G. Functional studies on macrophage populations in the airways and the lung wall of SPF mice in the steady-state and during respiratory virus infection. Immunology. 1988 Nov;65(3):417–425. [PMC free article] [PubMed] [Google Scholar]
- Breel M., Van der Ende M., Sminia T., Kraal G. Subpopulations of lymphoid and non-lymphoid cells in bronchus-associated lymphoid tissue (BALT) of the mouse. Immunology. 1988 Apr;63(4):657–662. [PMC free article] [PubMed] [Google Scholar]
- Chouaib S., Welte K., Mertelsmann R., Dupont B. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985 Aug;135(2):1172–1179. [PubMed] [Google Scholar]
- Curtis J. L., Kaltreider H. B. Characterization of bronchoalveolar lymphocytes during a specific antibody-forming cell response in the lungs of mice. Am Rev Respir Dis. 1989 Feb;139(2):393–400. doi: 10.1164/ajrccm/139.2.393. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Freundlich B. Human alveolar macrophage and blood monocyte inhibition of fibroblast proliferation. Evidence for synergy between interleukin-1 and tumor necrosis factor. Am Rev Respir Dis. 1988 Dec;138(6):1595–1603. doi: 10.1164/ajrccm/138.6.1595. [DOI] [PubMed] [Google Scholar]
- Elliott D. E., Righthand V. F., Boros D. L. Characterization of regulatory (interferon-alpha/beta) and accessory (LAF/IL 1) monokine activities from liver granuloma macrophages of Schistosoma mansoni-infected mice. J Immunol. 1987 Apr 15;138(8):2653–2662. [PubMed] [Google Scholar]
- Ellner J. J., Wallis R. S. Immunologic aspects of mycobacterial infections. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S455–S459. doi: 10.1093/clinids/11.supplement_2.s455. [DOI] [PubMed] [Google Scholar]
- Ettensohn D. B., Lalor P. A., Roberts N. J., Jr Human alveolar macrophage suppression of lymphocyte proliferation. Accessory characteristics for the generation and functional expression of con A-induced suppressor cells. Am Rev Respir Dis. 1988 Apr;137(4):765–773. doi: 10.1164/ajrccm/137.4.765. [DOI] [PubMed] [Google Scholar]
- Ferrick D. A., Herscowitz H. B. Cell interactions in alveolar macrophage-mediated suppression of the immune response: an unusual suppressor pathway involving a population of T-cells that express Lyt-1, L3T4, and I-J. Cell Immunol. 1988 Oct 1;116(1):183–194. doi: 10.1016/0008-8749(88)90220-1. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., O'Leary C., Krska K., Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985 Dec;62(3):586–593. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Degebrodt A., Venaille T., O'Leary C., Krska K., Flexman J., Farrell H., Shellam G., Young P., Penhale J. Preparation of interstitial lung cells by enzymatic digestion of tissue slices: preliminary characterization by morphology and performance in functional assays. Immunology. 1985 Jan;54(1):139–147. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol. 1986 Feb;63(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Robinson B. W., Reid M., Kees U. R., Warton A., Dawson V. H., Rose A., Schon-Hegrad M., Papadimitriou J. M. Extraction of immune and inflammatory cells from human lung parenchyma: evaluation of an enzymatic digestion procedure. Clin Exp Immunol. 1986 Oct;66(1):188–200. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Schon-Hegrad M. A., Oliver J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J Exp Med. 1988 Feb 1;167(2):262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. Immunogenicity signals 1,2,3 ... and 0. Immunol Today. 1989 Sep;10(9):283–286. doi: 10.1016/0167-5699(89)90081-9. [DOI] [PubMed] [Google Scholar]
- Kleinhenz M. E., Ellner J. J. Antigen responsiveness during tuberculosis: regulatory interactions of T cell subpopulations and adherent cells. J Lab Clin Med. 1987 Jul;110(1):31–40. [PubMed] [Google Scholar]
- Kobayashi K., Allred C., Castriotta R., Yoshida T. Strain variation of bacillus Calmette-Guerin-induced pulmonary granuloma formation is correlated with anergy and the local production of migration inhibition factor and interleukin 1. Am J Pathol. 1985 May;119(2):223–235. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Allred C., Cohen S., Yoshida T. Role of interleukin 1 in experimental pulmonary granuloma in mice. J Immunol. 1985 Jan;134(1):358–364. [PubMed] [Google Scholar]
- Kobayashi K., Allred C., Yoshida T. Mechanisms of suppressed cell-mediated immunity and impaired antigen-induced interleukin 2 production in granuloma-bearing mice. J Immunol. 1985 Nov;135(5):2996–3003. [PubMed] [Google Scholar]
- Kyes S., Carew E., Carding S. R., Janeway C. A., Jr, Hayday A. Diversity in T-cell receptor gamma gene usage in intestinal epithelium. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5527–5531. doi: 10.1073/pnas.86.14.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft B. J., Pedrotti P. W., Remington J. S. In vitro generation of adherent mononuclear suppressor cells to Toxoplasma antigen. Immunology. 1988 Apr;63(4):643–648. [PMC free article] [PubMed] [Google Scholar]
- Mbawuike I. N., Herscowitz H. B. Role of activation in alveolar macrophage-mediated suppression of the plaque-forming cell response. Infect Immun. 1988 Mar;56(3):577–581. doi: 10.1128/iai.56.3.577-581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B. The I-J puzzle. Annu Rev Immunol. 1987;5:405–427. doi: 10.1146/annurev.iy.05.040187.002201. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Sileghem M., Darji A., Remels L., Hamers R., De Baetselier P. Different mechanisms account for the suppression of interleukin 2 production and the suppression of interleukin 2 receptor expression in Trypanosoma brucei-infected mice. Eur J Immunol. 1989 Jan;19(1):119–124. doi: 10.1002/eji.1830190119. [DOI] [PubMed] [Google Scholar]
- Toossi Z., Kleinhenz M. E., Ellner J. J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986 May 1;163(5):1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Dougherty S., Evequoz V., Pluznik D. H., Wilder R. L., Hand A. R., Wahl L. M. T lymphocyte-dependent evolution of bacterial cell wall-induced hepatic granulomas. J Immunol. 1986 Oct 1;137(7):2199–2209. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Bansal G., McCartney-Francis N., Ellingsworth L., Allen J. B. Bacterial cell wall-induced immunosuppression. Role of transforming growth factor beta. J Exp Med. 1988 Oct 1;168(4):1403–1417. doi: 10.1084/jem.168.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltenbaugh C., Sun L., Lei H. Y. Regulation of immune responses by I-J gene products. VI. Recognition of I-E molecules by I-J-bearing suppressor factors. J Exp Med. 1986 Apr 1;163(4):797–811. doi: 10.1084/jem.163.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]


