Abstract
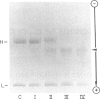
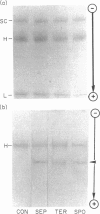
Three bacterial species of Clostridium (septicum, tertium and sporogenes) were identified to produce extracellular proteases cleaving IgA to Fab and Fc fragments, as demonstrated by SDS-PAGE and immunoelectrophoretic procedures. These enzymes acted on monometric IgA1 paraproteins and normal serum IgA1 but had no activity on IgA2 paraproteins and intact secretory IgA1 from human colostrum. Their action on polyclonal serum IgA1 suggested the absence of neutralizing anti-clostridial IgA protease activity. Although the enzymes were shown not to act on secretory IgA1, they were, however, able to digest free alpha-heavy chains of the dimeric IgA molecules. Susceptibility of the alpha-heavy chain to the proteases was more likely due to the change to a more accessible conformation than because of the absence of neutralizing anti-enzymic activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujiyama Y., Iwaki M., Hodohara K., Hosoda S., Kobayashi K. The site of cleavage in human alpha chains of IgA1 and IgA2:A2m(1) allotype paraproteins by the clostridial IGA protease. Mol Immunol. 1986 Feb;23(2):147–150. doi: 10.1016/0161-5890(86)90036-2. [DOI] [PubMed] [Google Scholar]
- Fujiyama Y., Kobayashi K., Senda S., Benno Y., Bamba T., Hosoda S. A novel IgA protease from Clostridium sp. capable of cleaving IgA1 and IgA2 A2m(1) but not IgA2 A2m(2) allotype paraproteins. J Immunol. 1985 Jan;134(1):573–576. [PubMed] [Google Scholar]
- Gilbert J. V., Plaut A. G., Longmaid B., Lamm M. E. Inhibition of microbial IgA proteases by human secretory IgA and serum. Mol Immunol. 1983 Sep;20(9):1039–1049. doi: 10.1016/0161-5890(83)90045-7. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Russell M. W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988 Jun;52(2):296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Fujiyama Y., Hagiwara K., Kondoh H. Resistance of normal serum IgA and secretory IgA to bacterial IgA proteases: evidence for the presence of enzyme-neutralizing antibodies in both serum and secretory IgA, and also in serum IgG. Microbiol Immunol. 1987;31(11):1097–1106. doi: 10.1111/j.1348-0421.1987.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Male C. J. Immunoglobulin A1 protease production by Haemophilus influenzae and Streptococcus pneumoniae. Infect Immun. 1979 Oct;26(1):254–261. doi: 10.1128/iai.26.1.254-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansa B., Kilian M. Retained antigen-binding activity of Fab alpha fragments of human monoclonal immunoglobulin A1 (IgA1) cleaved by IgA1 protease. Infect Immun. 1986 Apr;52(1):171–174. doi: 10.1128/iai.52.1.171-174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulks M. H., Plaut A. G., Feldman H. A., Frangione B. IgA proteases of two distinct specificities are released by Neisseria meningitidis. J Exp Med. 1980 Nov 1;152(5):1442–1447. doi: 10.1084/jem.152.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]