Abstract
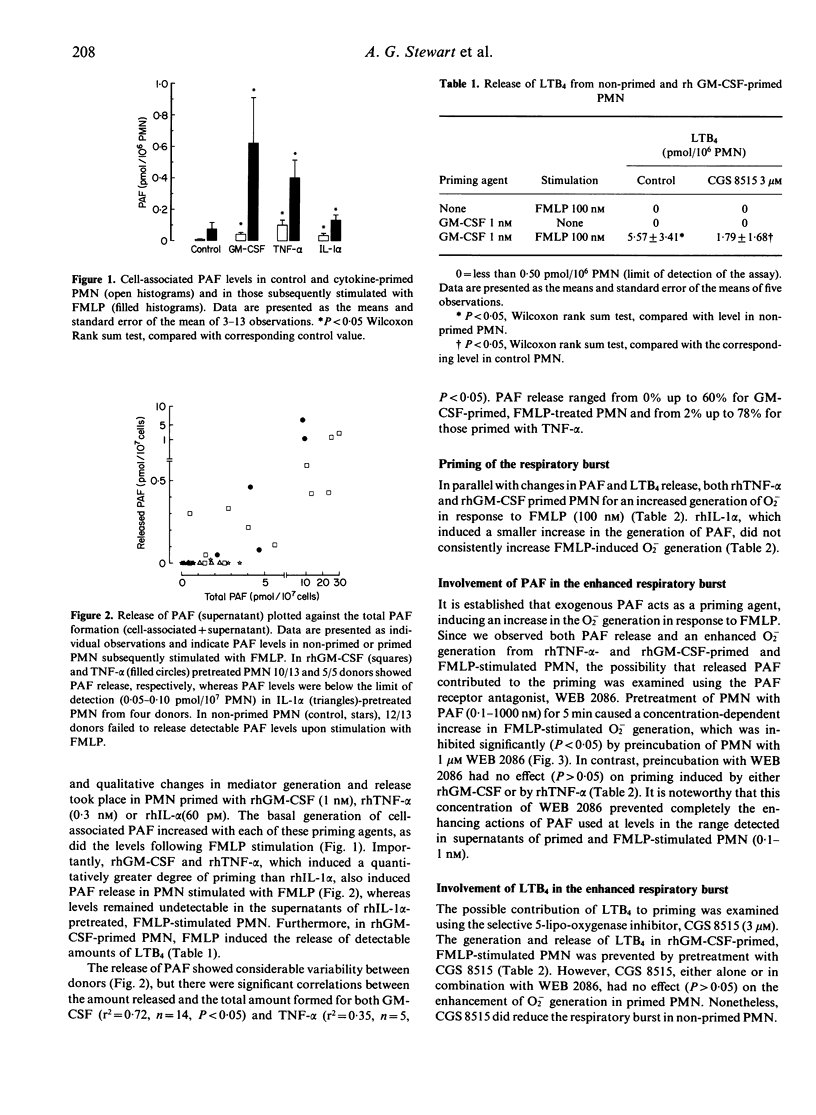
Recombinant human (rh) tumour necrosis factor (TNF) alpha and rh granulocyte-macrophage colony-stimulating factor (GM-CSF) prime human polymorphonuclear leucocytes (PMN) for increased superoxide anion (O2-) generation and for increased platelet-activating factor (PAF) biosynthesis and leukotriene B4 (LTB4) release. Both PAF and LTB4 are candidate mediators for the enhanced O2- generation in cytokine-primed PMN, since exogenous PAF or LTB4 primes PMN. We measured the generation and release of these mediators and examined their potential roles in cytokine priming using the PAF receptor antagonist, WEB 2086, and the inhibitor of 5-lipo-oxygenase, CGS 8515.rhTNF-alpha or rhGM-CSF, alone, increased PAF levels in PMN, but did not cause PAF release or LTB4 synthesis. N-formylmethionyl-leucyl-phenylalanine (FMLP) stimulated the release of detectable and biologically active amounts of both LTB4 and PAF in primed, but not in non-primed PMN. However, neither blockade of PAF receptors, nor inhibition of LTB4 synthesis influenced the priming of O2- generation by rhTNF-alpha or rhGM-CSF. Simultaneous pretreatment of PMN with WEB 2086 and CGS 8515 also failed to inhibit priming. Our results do not exclude a role for cell-associated PAF in the priming response, but indicate that the release of PAF and LTB4 do not mediate this phenomenon. The ability of cytokines to amplify the production and release of lipids may represent a mechanism to attract and localize the pro-inflammatory actions of stimulated PMN to regions where cytokine levels are also elevated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson Y. H., Lopez A. F., Marasco W. A., Lucas C. M., Wong G. G., Burns G. F., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor (rH GM-CSF) regulates f Met-Leu-Phe receptors on human neutrophils. Immunology. 1988 Jul;64(3):519–525. [PMC free article] [PubMed] [Google Scholar]
- Atkinson Y. H., Marasco W. A., Lopez A. F., Vadas M. A. Recombinant human tumor necrosis factor-alpha. Regulation of N-formylmethionylleucylphenylalanine receptor affinity and function on human neutrophils. J Clin Invest. 1988 Mar;81(3):759–765. doi: 10.1172/JCI113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson Y. H., Murray A. W., Krilis S., Vadas M. A., Lopez A. F. Human tumour necrosis factor-alpha (TNF-alpha) directly stimulates arachidonic acid release in human neutrophils. Immunology. 1990 May;70(1):82–87. [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B. Stimulus amplification by PAF and LTB4 in human neutrophils. Pharmacol Res Commun. 1986 Aug;18 (Suppl):51–59. doi: 10.1016/0031-6989(86)90038-x. [DOI] [PubMed] [Google Scholar]
- Braquet P., Paubert-Braquet M., Bourgain R. H., Bussolino F., Hosford D. PAF/cytokine auto-generated feedback networks in microvascular immune injury: consequences in shock, ischemia and graft rejection. J Lipid Mediat. 1989 Mar-Apr;1(2):75–112. [PubMed] [Google Scholar]
- Braquet P., Rola-Pleszczynski M. The role of PAF in immunological responses: a review. Prostaglandins. 1987 Aug;34(2):143–148. doi: 10.1016/0090-6980(87)90190-0. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Breviario F., Tetta C., Aglietta M., Mantovani A., Dejana E. Interleukin 1 stimulates platelet-activating factor production in cultured human endothelial cells. J Clin Invest. 1986 Jun;77(6):2027–2033. doi: 10.1172/JCI112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Bussolino F., Baglioni C. Tumor necrosis factor stimulates human neutrophils to release leukotriene B4 and platelet-activating factor. Induction of phospholipase A2 and acetyl-CoA:1-alkyl-sn-glycero-3-phosphocholine O2-acetyltransferase activity and inhibition by antiproteinase. Eur J Biochem. 1989 Jul 1;182(3):661–666. doi: 10.1111/j.1432-1033.1989.tb14876.x. [DOI] [PubMed] [Google Scholar]
- Casals-Stenzel J., Muacevic G., Weber K. H. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther. 1987 Jun;241(3):974–981. [PubMed] [Google Scholar]
- Cluzel M., Undem B. J., Chilton F. H. Release of platelet-activating factor and the metabolism of leukotriene B4 by the human neutrophil when studied in a cell superfusion model. J Immunol. 1989 Dec 1;143(11):3659–3665. [PubMed] [Google Scholar]
- Coffey R. G., Davis J. S., Djeu J. Y. Stimulation of guanylate cyclase activity and reduction of adenylate cyclase activity by granulocyte-macrophage colony-stimulating factor in human blood neutrophils. J Immunol. 1988 Apr 15;140(8):2695–2701. [PubMed] [Google Scholar]
- Corey S. J., Rosoff P. M. Granulocyte-macrophage colony-stimulating factor primes neutrophils by activating a pertussis toxin-sensitive G protein not associated with phosphatidylinositol turnover. J Biol Chem. 1989 Aug 25;264(24):14165–14171. [PubMed] [Google Scholar]
- Dahinden C. A., Zingg J., Maly F. E., de Weck A. L. Leukotriene production in human neutrophils primed by recombinant human granulocyte/macrophage colony-stimulating factor and stimulated with the complement component C5A and FMLP as second signals. J Exp Med. 1988 Apr 1;167(4):1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J. F., Naccache P. H., Borgeat P., Gasson J. C., Nguyen M. H., McColl S. R. Characterization of the priming effects of human granulocyte-macrophage colony-stimulating factor on human neutrophil leukotriene synthesis. Prostaglandins. 1988 Nov;36(5):673–691. doi: 10.1016/0090-6980(88)90013-5. [DOI] [PubMed] [Google Scholar]
- Doerfler M. E., Danner R. L., Shelhamer J. H., Parrillo J. E. Bacterial lipopolysaccharides prime human neutrophils for enhanced production of leukotriene B4. J Clin Invest. 1989 Mar;83(3):970–977. doi: 10.1172/JCI113983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Holden C. S., Humphreys J. M., Hart C. A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) primes the respiratory burst and stimulates protein biosynthesis in human neutrophils. FEBS Lett. 1989 Oct 9;256(1-2):62–66. doi: 10.1016/0014-5793(89)81718-1. [DOI] [PubMed] [Google Scholar]
- Gay J. C., Beckman J. K., Zaboy K. A., Lukens J. N. Modulation of neutrophil oxidative responses to soluble stimuli by platelet-activating factor. Blood. 1986 Apr;67(4):931–936. [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Ku E. C., Raychaudhuri A., Ghai G., Kimble E. F., Lee W. H., Colombo C., Dotson R., Oglesby T. D., Wasley J. W. Characterization of CGS 8515 as a selective 5-lipoxygenase inhibitor using in vitro and in vivo models. Biochim Biophys Acta. 1988 Apr 15;959(3):332–342. doi: 10.1016/0005-2760(88)90207-x. [DOI] [PubMed] [Google Scholar]
- Leyravaud S., Benveniste J. Regulation of cellular retention of paf-acether by extracellular pH and cell concentration. Biochim Biophys Acta. 1989 Sep 25;1005(2):192–195. doi: 10.1016/0005-2760(89)90187-2. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Williamson D. J., Gamble J. R., Begley C. G., Harlan J. M., Klebanoff S. J., Waltersdorph A., Wong G., Clark S. C., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986 Nov;78(5):1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. M., Henson P. M. The intracellular retention of newly synthesized platelet-activating factor. J Immunol. 1986 Oct 15;137(8):2653–2661. [PubMed] [Google Scholar]
- McColl S. R., Krump E., Naccache P. H., Borgeat P. Enhancement of human neutrophil leukotriene synthesis by human granulocyte-macrophage colony-stimulating factor. Agents Actions. 1989 Jun;27(3-4):465–468. doi: 10.1007/BF01972854. [DOI] [PubMed] [Google Scholar]
- Mege J. L., Gomez-Cambronero J., Molski T. F., Becker E. L., Sha'afi R. I. Effect of granulocyte-macrophage colony-stimulating factor on superoxide production in cytoplasts and intact human neutrophils: role of protein kinase and G-proteins. J Leukoc Biol. 1989 Aug;46(2):161–168. doi: 10.1002/jlb.46.2.161. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Faucher N., Borgeat P., Gasson J. C., DiPersio J. F. Granulocyte-macrophage colony-stimulating factor modulates the excitation-response coupling sequence in human neutrophils. J Immunol. 1988 May 15;140(10):3541–3546. [PubMed] [Google Scholar]
- O'Flaherty J. T. Phospholipid metabolism and stimulus-response coupling. Biochem Pharmacol. 1987 Feb 15;36(4):407–412. doi: 10.1016/0006-2952(87)90343-1. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Salmon J. A. Release of leukotriene B4 from human neutrophils and its relationship to degranulation induced by N-formyl-methionyl-leucyl-phenylalanine, serum-treated zymosan and the ionophore A23187. Immunology. 1983 Sep;50(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Poubelle P. E., Bourgoin S., Naccache P. H., Borgeat P. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and opsonization synergistically enhance leukotriene B4 (LTB4) synthesis induced by phagocytosis in human neutrophils. Agents Actions. 1989 Jun;27(3-4):388–390. doi: 10.1007/BF01972830. [DOI] [PubMed] [Google Scholar]
- Salmon J. A., Simmons P. M., Palmer R. M. A radioimmunoassay for leukotriene B4. Prostaglandins. 1982 Aug;24(2):225–235. doi: 10.1016/0090-6980(82)90148-4. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Naccache P. H., Sha'afi R. I., Becker E. L. Inhibition of rabbit neutrophil lysosomal enzyme secretion, non-stimulated and chemotactic factor stimulated locomotion by nordihydroguaiaretic acid. Life Sci. 1980 Aug 4;27(5):421–426. doi: 10.1016/0024-3205(80)90191-5. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Weissmann G. Effects of indomethacin, 5,8,11,14-eicosatetraynoic acid, and p-bromophenacyl bromide on lysosomal enzyme release and superoxide anion generation by human polymorphonuclear leukocytes. Biochem Pharmacol. 1980 Feb 15;29(4):533–538. doi: 10.1016/0006-2952(80)90373-1. [DOI] [PubMed] [Google Scholar]
- Stewart A. G., Dubbin P. N., Harris T., Dusting G. J. Evidence for an intracellular action of platelet-activating factor in bovine cultured aortic endothelial cells. Br J Pharmacol. 1989 Mar;96(3):503–505. doi: 10.1111/j.1476-5381.1989.tb11845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. G., Dubbin P. N., Harris T., Dusting G. J. Platelet-activating factor may act as a second messenger in the release of icosanoids and superoxide anions from leukocytes and endothelial cells. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3215–3219. doi: 10.1073/pnas.87.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. G., Dusting G. J. Characterization of receptors for platelet-activating factor on platelets, polymorphonuclear leukocytes and macrophages. Br J Pharmacol. 1988 Aug;94(4):1225–1233. doi: 10.1111/j.1476-5381.1988.tb11642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. G., Phillips W. A. Intracellular platelet-activating factor regulates eicosanoid generation in guinea-pig resident peritoneal macrophages. Br J Pharmacol. 1989 Sep;98(1):141–148. doi: 10.1111/j.1476-5381.1989.tb16874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Sullivan J. A., Murata T., Mandell G. L. Both recombinant interleukin-1 (beta) and purified human monocyte interleukin-1 prime human neutrophils for increased oxidative activity and promote neutrophil spreading. J Leukoc Biol. 1989 May;45(5):389–395. doi: 10.1002/jlb.45.5.389. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Fredette J. P., Griffin J. D., Leavitt J. L., Simons E. R., Melnick D. A. An elevation in the concentration of free cytosolic calcium is sufficient to activate the oxidative burst of granulocytes primed with recombinant human granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1989 Apr 15;264(11):6302–6309. [PubMed] [Google Scholar]
- Sullivan R., Fredette J. P., Leavitt J. L., Gadenne A. S., Griffin J. D., Simons E. R. Effects of recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSFrh) on transmembrane electrical potentials in granulocytes: relationship between enhancement of ligand-mediated depolarization and augmentation of superoxide anion (O2-) production. J Cell Physiol. 1989 May;139(2):361–369. doi: 10.1002/jcp.1041390219. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Griffin J. D., Simons E. R., Schafer A. I., Meshulam T., Fredette J. P., Maas A. K., Gadenne A. S., Leavitt J. L., Melnick D. A. Effects of recombinant human granulocyte and macrophage colony-stimulating factors on signal transduction pathways in human granulocytes. J Immunol. 1987 Nov 15;139(10):3422–3430. [PubMed] [Google Scholar]
- Tool A. T., Verhoeven A. J., Roos D., Koenderman L. Platelet-activating factor (PAF) acts as an intercellular messenger in the changes of cytosolic free Ca2+ in human neutrophils induced by opsonized particles. FEBS Lett. 1989 Dec 18;259(1):209–212. doi: 10.1016/0014-5793(89)81530-3. [DOI] [PubMed] [Google Scholar]
- Vargas J. R., Radomski M., Moncada S. The use of prostacyclin in the separation from plasma and washing of human platelets. Prostaglandins. 1982 Jun;23(6):929–945. doi: 10.1016/0090-6980(82)90135-6. [DOI] [PubMed] [Google Scholar]
- Walenga R. W., Showell H. J., Feinstein M. B., Becker E. L. Parallel inhibition of neutrophil arachidonic acid metabolism and lysosomal enzyme secretion by nordihydroguaiaretic acid. Life Sci. 1980 Sep 22;27(12):1047–1053. doi: 10.1016/0024-3205(80)90028-4. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Williams J. D., Lee T. H., Lewis R. A., Austen F. Intracellular retention of the 5-lipoxygenase pathway product, leukotriene B4, by human neutrophils activated with unopsonized zymosan. J Immunol. 1985 Apr;134(4):2624–2630. [PubMed] [Google Scholar]
- Wirthmueller U., De Weck A. L., Dahinden C. A. Platelet-activating factor production in human neutrophils by sequential stimulation with granulocyte-macrophage colony-stimulating factor and the chemotactic factors C5A or formyl-methionyl-leucyl-phenylalanine. J Immunol. 1989 May 1;142(9):3213–3218. [PubMed] [Google Scholar]
- Worthen G. S., Seccombe J. F., Clay K. L., Guthrie L. A., Johnston R. B., Jr The priming of neutrophils by lipopolysaccharide for production of intracellular platelet-activating factor. Potential role in mediation of enhanced superoxide secretion. J Immunol. 1988 May 15;140(10):3553–3559. [PubMed] [Google Scholar]



