Abstract
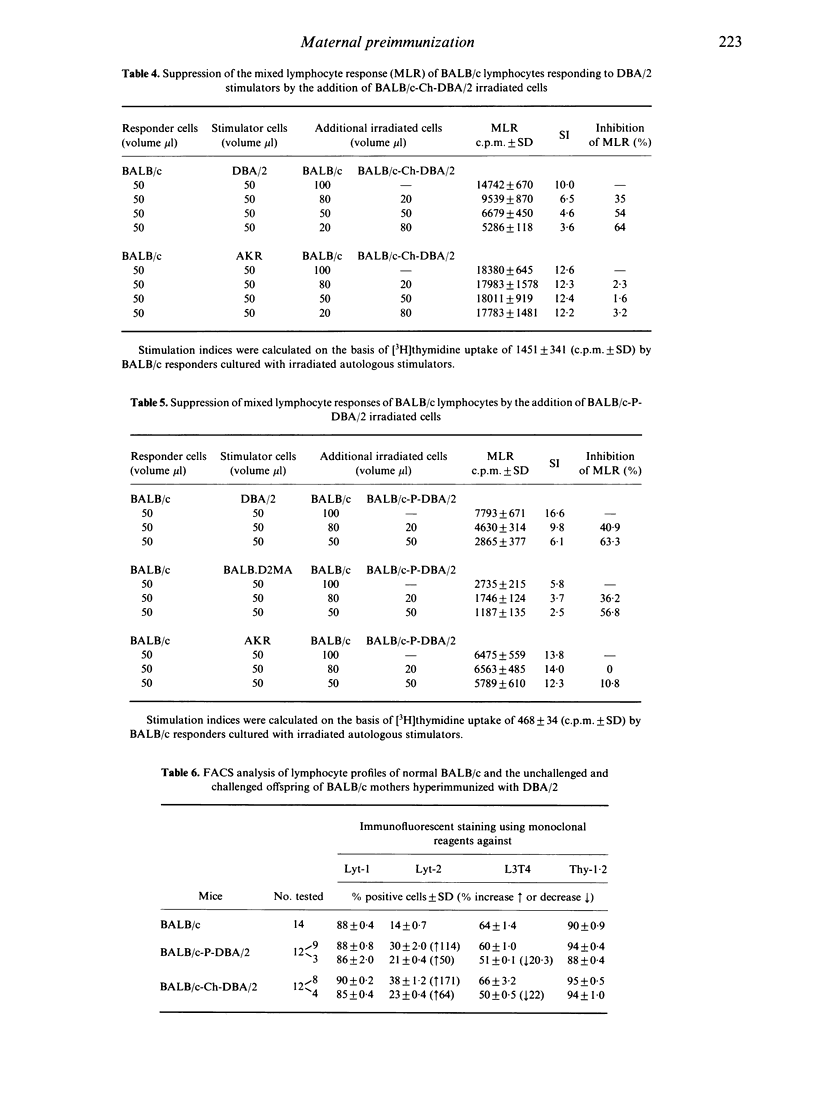
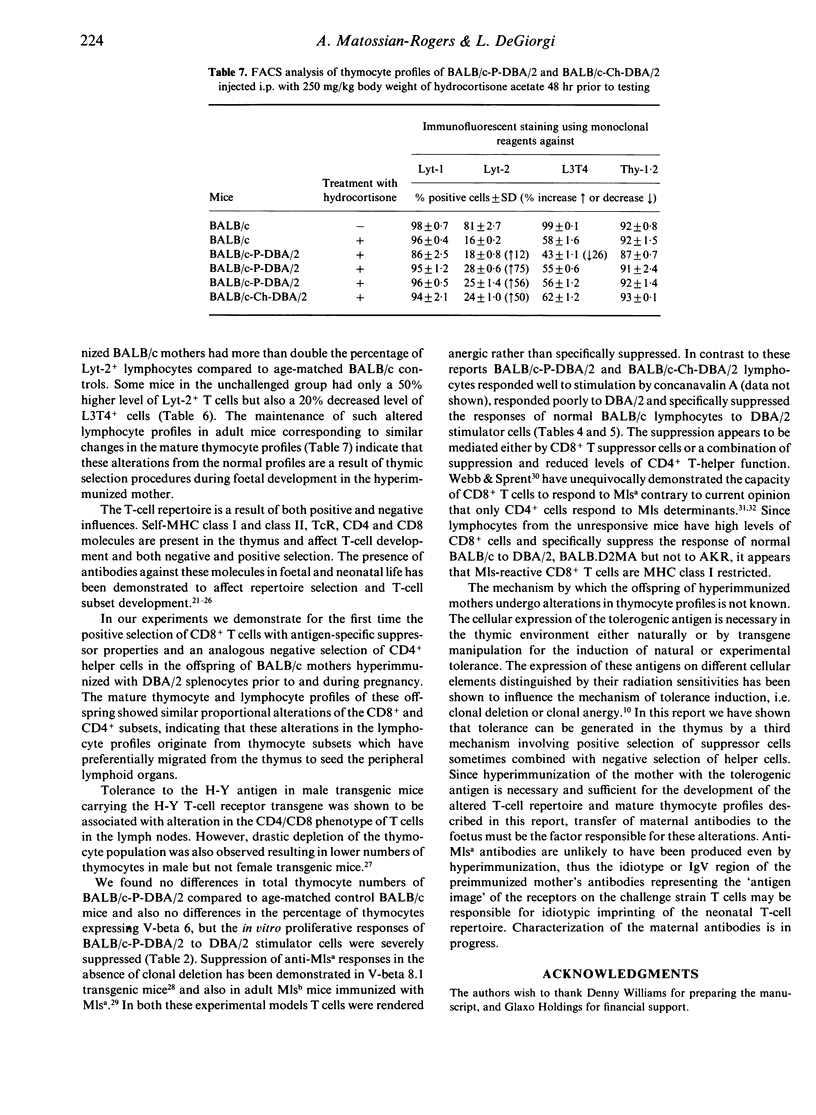
Female BALB/c (H-2d, Mlsb) mice alloimmunized prior to and during syngeneic pregnancy with DBA/2 (H-2d, Mlsa) splenocytes gave rise to offspring with severely reduced responsiveness in adult life to DBA/2 stimulation in vitro mixed lymphocyte cultures. The offspring of the hyperimmunized mothers were also tolerant to neonatal challenge with large numbers of DBA/2 splenocytes, which resulted in runting disease of control neonatal BALB/c mice. Both challenged and unchallenged offspring of the immunized BALB/c mothers were hyporesponsive to DBA/2 but both stimulated and responded to normal BALB/c lymphocytes, indicating alteration in their T-cell repertoire. There was no reduction in the V beta 6-positive thymocyte subpopulation in the challenged or unchallenged offspring of the alloimmunized BALB/c mothers compared to normal controls, suggesting that hyporesponsiveness to DBA/2 is not due to thymic deletion of Mlsa-responsive clones. Examination of the T-cell subset composition of the hydrocortisone-resistant thymocytes and peripheral lymphocytes of the challenged and unchallenged groups of experimental mice revealed large increases in the percentage of Lyt-2+ T cells, sometimes accompanied by a decrease in the L3T4+ T-cell subset compared to age-matched control BALB/c. Lymphocytes from the hyporesponsive mice specifically suppressed the proliferative responses of control BALB/c to DBA/2 but not to AKR. The data indicate that maternal hyperimmunization can induce tolerance by a mechanism involving intrathymic selection of suppressor cells which can be combined with a negative selection of helper cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbasi K., Démant P., Festenstein H., Holmes J., Huber B., Rychiková M. Mouse mixed lymphocyte reactions and cell-mediated lympholysis: genetic control and relevance to antigenic strength. Transplant Proc. 1973 Dec;5(4):1329–1337. [PubMed] [Google Scholar]
- Abe R., Hodes R. J. T-cell recognition of minor lymphocyte stimulating (Mls) gene products. Annu Rev Immunol. 1989;7:683–708. doi: 10.1146/annurev.iy.07.040189.003343. [DOI] [PubMed] [Google Scholar]
- Abe R., Vacchio M. S., Fox B., Hodes R. J. Preferential expression of the T-cell receptor V beta 3 gene by Mlsc reactive T cells. Nature. 1988 Oct 27;335(6193):827–830. doi: 10.1038/335827a0. [DOI] [PubMed] [Google Scholar]
- Bandeira A., Coutinho A., Carnaud C., Jacquemart F., Forni L. Transplantation tolerance correlates with high levels of T- and B-lymphocyte activity. Proc Natl Acad Sci U S A. 1989 Jan;86(1):272–276. doi: 10.1073/pnas.86.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Burgert H. G., Gerhard-Burgert H., Woodland D. L., Palmer E., Kappler J. W., Marrack P. A role for clonal inactivation in T cell tolerance to Mls-1a. Nature. 1990 Jun 7;345(6275):540–542. doi: 10.1038/345540a0. [DOI] [PubMed] [Google Scholar]
- Bonneville M., Ishida I., Itohara S., Verbeek S., Berns A., Kanagawa O., Haas W., Tonegawa S. Self-tolerance to transgenic gamma delta T cells by intrathymic inactivation. Nature. 1990 Mar 8;344(6262):163–165. doi: 10.1038/344163a0. [DOI] [PubMed] [Google Scholar]
- DeGiorgi L., Habeshaw J. A., Povey S., Matossian-Rogers A. Reduction of graft-versus-host disease in neonatal F1 hybrid mice. Clin Exp Immunol. 1990 Jan;79(1):130–134. doi: 10.1111/j.1365-2249.1990.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgi L., Matossian-Rogers A., Festenstein H. Protection of newborn mice from graft versus host disease by maternal pre-immunization. J Immunogenet. 1990 Feb-Apr;17(1-2):77–88. doi: 10.1111/j.1744-313x.1990.tb00861.x. [DOI] [PubMed] [Google Scholar]
- DeGiorgi L., Povey S., Habeshaw J. A., Davies A. J. Prevention of graft-versus-host disease in neonatal F1 hybrid mice by pre-immunisation of their mother with paternal spleen cells. Transplant Proc. 1989 Feb;21(1 Pt 3):3045–3049. [PubMed] [Google Scholar]
- Eto M., Mayumi H., Tomita Y., Yoshikai Y., Nomoto K. Intrathymic clonal deletion of V beta 6+ T cells in cyclophosphamide-induced tolerance to H-2-compatible, Mls-disparate antigens. J Exp Med. 1990 Jan 1;171(1):97–113. doi: 10.1084/jem.171.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A. M., Matis L. A. Self-tolerance alters T-cell receptor expression in an antigen-specific MHC restricted immune response. Nature. 1988 Oct 27;335(6193):830–832. doi: 10.1038/335830a0. [DOI] [PubMed] [Google Scholar]
- Haqqi T. M., Banerjee S., Anderson G. D., David C. S. RIII S/J (H-2r). An inbred mouse strain with a massive deletion of T cell receptor V beta genes. J Exp Med. 1989 Jun 1;169(6):1903–1909. doi: 10.1084/jem.169.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kruisbeek A. M., Mond J. J., Fowlkes B. J., Carmen J. A., Bridges S., Longo D. L. Absence of the Lyt-2-,L3T4+ lineage of T cells in mice treated neonatally with anti-I-A correlates with absence of intrathymic I-A-bearing antigen-presenting cell function. J Exp Med. 1985 May 1;161(5):1029–1047. doi: 10.1084/jem.161.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao N. S., Maltzman J., Raulet D. H. Positive selection determines T cell receptor V beta 14 gene usage by CD8+ T cells. J Exp Med. 1989 Jul 1;170(1):135–143. doi: 10.1084/jem.170.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K. Programmed death of autoreactive thymocytes. Nature. 1990 Feb 15;343(6259):642–644. doi: 10.1038/343642a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Lo D., Brinster R., Palmiter R., Burkly L., Flavell R. H., Kappler J. The effect of thymus environment on T cell development and tolerance. Cell. 1988 May 20;53(4):627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- Maruŝić-Galesić S., Stephany D. A., Longo D. L., Kruisbeek A. M. Development of CD4-CD8+ cytotoxic T cells requires interactions with class I MHC determinants. Nature. 1988 May 12;333(6169):180–183. doi: 10.1038/333180a0. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Kroschewski R., Frangoulis B. Clonal anergy induced in mature V beta 6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989 Jun 15;339(6225):541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Sharrow S. O., Singer A. Clonal deletion and clonal anergy in the thymus induced by cellular elements with different radiation sensitivities. J Exp Med. 1990 Mar 1;171(3):935–940. doi: 10.1084/jem.171.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Lees R. K., Pedrazzini T., Zinkernagel R. M., Hengartner H., MacDonald H. R. Postnatal disappearance of self-reactive (V beta 6+) cells from the thymus of Mlsa mice. Implications for T cell development and autoimmunity. J Exp Med. 1989 Jun 1;169(6):2149–2158. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser D. E., Schneider R., Hengartner H., MacDonald H. R., Zinkernagel R. M. Clonal deletion of self-reactive T cells in irradiation bone marrow chimeras and neonatally tolerant mice. Evidence for intercellular transfer of Mlsa. J Exp Med. 1989 Aug 1;170(2):595–600. doi: 10.1084/jem.170.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. R., Sprent J. Response of mature unprimed CD8+ T cells to Mlsa determinants. J Exp Med. 1990 Mar 1;171(3):953–958. doi: 10.1084/jem.171.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúiga-Pflücker J. C., Jones L. A., Longo D. L., Kruisbeek A. M. CD8 is required during positive selection of CD4-/CD8+ T cells. J Exp Med. 1990 Feb 1;171(2):427–437. doi: 10.1084/jem.171.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]



