Abstract
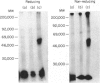
The guinea-pig (gp) CD4 protein was identified using the rat monoclonal antibody (mAb) H155 derived from an interspecies hybrid. The hybrid was obtained after immunization of rats with purified guinea-pig T lymphocytes and fusion to a mouse myeloma line. The mAb H155 reacted with a subpopulation (60-70%) of nylon-wool-purified guinea-pig T cells. The majority of thymocytes also expressed the H155 antigen. Immunohistological staining showed that predominantly the cortical thymocytes were H155-positive, whereas a part of the medullary cells did not express the antigen. After cell-surface radioiodination of T cell lines, the mAb H155 immunoprecipitated a molecule of about 55,000 MW both under reducing and non-reducing conditions. The antigen recognized by mAb H155 on guinea-pig T cells resembles the human or mouse CD4 antigen. Depletion experiments in combination with functional studies further supported this assumption. In proliferation assays mAb H155 inhibited T-cell responses in vitro. Both the antigen- and alloantigen-induced T-cell activation were impaired in the continuous presence of mAb H155. In addition, mAb H155 inhibited both the mitogen-induced T-cell activation and T-cell proliferation induced by mitogenic mAb and phorbol myristate acetate (PMA). Binding of the mAb H155 to the CD4 molecule might therefore transduce a negative signal on T-cell activation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthos J., Deen K. C., Chaikin M. A., Fornwald J. A., Sathe G., Sattentau Q. J., Clapham P. R., Weiss R. A., McDougal J. S., Pietropaolo C. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989 May 5;57(3):469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- Auerbach H. S., Baker R. D., Matthews W. J., Jr, Colten H. R. Molecular mechanism for feedback regulation of C4 biosynthesis in guinea pig peritoneal macrophage. J Exp Med. 1984 Jun 1;159(6):1750–1761. doi: 10.1084/jem.159.6.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber E. K., Dasgupta J. D., Schlossman S. F., Trevillyan J. M., Rudd C. E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989 May;86(9):3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin H., Xhurdebise L. M., Burtonboy G., Lebacq A. M., De Clercq L., Cormont F. Rat monoclonal antibodies. I. Rapid purification from in vitro culture supernatants. J Immunol Methods. 1984 Feb 10;66(2):261–269. doi: 10.1016/0022-1759(84)90337-5. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Reinherz E. L., Poppema S., McCluskey R. T., Schlossman S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med. 1980 Oct 1;152(4):771–782. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bia F. J., Lucia H. L., Fong C. K., Tarsio M., Hsiung G. D. Effects of vaccination on cytomegalovirus-associated interstitial pneumonia in strain 2 guinea pigs. J Infect Dis. 1982 May;145(5):742–747. doi: 10.1093/infdis/145.2.742. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Rao P. E., Talle M. A., Goldstein G., Shaw S. Possible involvement of the T4 molecule in T cell recognition of class II HLA antigens. Evidence from studies of CTL-target cell binding. J Exp Med. 1984 Mar 1;159(3):783–797. doi: 10.1084/jem.159.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R., Deubel U., Hadding U., Bitter-Suermann D. Identification of functionally relevant determinants on the complement component C3 with monoclonal antibodies. J Immunol. 1982 Nov;129(5):2042–2050. [PubMed] [Google Scholar]
- Burger R., Schrod L., Schaefer H. Functionally relevant membrane proteins of human and guinea-pig T lymphocytes. Mol Immunol. 1986 Nov;23(11):1149–1156. doi: 10.1016/0161-5890(86)90145-8. [DOI] [PubMed] [Google Scholar]
- Carrera A. C., Sanchez-Madrid F., Lopez-Botet M., Bernabeu C., De Landazuri M. O. Involvement of the CD4 molecule in a post-activation event on T cell proliferation. Eur J Immunol. 1987 Feb;17(2):179–186. doi: 10.1002/eji.1830170205. [DOI] [PubMed] [Google Scholar]
- Coulie P. G., Coutelier J. P., Uyttenhove C., Lambotte P., Van Snick J. In vivo suppression of T-dependent antibody responses by treatment with a monoclonal anti-L3T4 antibody. Eur J Immunol. 1985 Jun;15(6):638–640. doi: 10.1002/eji.1830150620. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Jefferies W. A., Clark S. J., Chung L. P., Gordon S. Species heterogeneity in macrophage expression of the CD4 antigen. J Exp Med. 1987 Aug 1;166(2):613–618. doi: 10.1084/jem.166.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Ermak T. H., Steger H. J., Wofsy D. Treatment of murine lupus with monoclonal antibody to L3T4. II. Effects on immunohistopathology of thymus, spleen, and lymph node. Lab Invest. 1989 Oct;61(4):447–456. [PubMed] [Google Scholar]
- Gaudernack G., Leivestad T., Ugelstad J., Thorsby E. Isolation of pure functionally active CD8+ T cells. Positive selection with monoclonal antibodies directly conjugated to monosized magnetic microspheres. J Immunol Methods. 1986 Jun 24;90(2):179–187. doi: 10.1016/0022-1759(86)90074-8. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Antigen processing and CD4+ T cell depletion in AIDS. Cell. 1988 Aug 12;54(4):441–444. doi: 10.1016/0092-8674(88)90062-1. [DOI] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J Exp Med. 1986 Sep 1;164(3):911–925. doi: 10.1084/jem.164.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Curtis J., Turk J. L. Accessory cell function of cells of the mononuclear phagocyte system isolated from mycobacterial granulomas. Cell Immunol. 1985 Apr 1;91(2):425–433. doi: 10.1016/0008-8749(85)90240-0. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Clayberger C. Diagnostic and therapeutic implications of T cell surface antigens. Transplantation. 1985 Apr;39(4):339–348. doi: 10.1097/00007890-198504000-00001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Molineaux S. M., Maddon D. E., Zimmerman K. A., Godfrey M., Alt F. W., Chess L., Axel R. Structure and expression of the human and mouse T4 genes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9155–9159. doi: 10.1073/pnas.84.24.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Endres R., Shimonkevitz R., Zlotnik A., Dialynas D., Fitch F., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983 Oct 1;158(4):1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P. D., Lassmann H., Wisniewski H. M. Immunologic studies of chronic relapsing EAE in guinea pigs: similarities to multiple sclerosis. J Immunol. 1981 Jul;127(1):334–338. [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Altman A. Do CD4 and CD8 control T-cell activation via a specific tyrosine protein kinase? Immunol Today. 1989 Jun;10(6):189–192. doi: 10.1016/0167-5699(89)90322-8. [DOI] [PubMed] [Google Scholar]
- Peterson A., Seed B. Genetic analysis of monoclonal antibody and HIV binding sites on the human lymphocyte antigen CD4. Cell. 1988 Jul 1;54(1):65–72. doi: 10.1016/0092-8674(88)90180-8. [DOI] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Romain P. L., Schlossman S. F. Human T lymphocyte subsets. Functional heterogeneity and surface recognition structures. J Clin Invest. 1984 Nov;74(5):1559–1565. doi: 10.1172/JCI111571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Weiss R. A. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988 Mar 11;52(5):631–633. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- Schäfer H., Baker D., Thiele B., Burger R. Structure, cellular distribution, and functional characteristics of the guinea pig leucocyte common antigen. Cell Immunol. 1990 Jul;128(2):370–384. doi: 10.1016/0008-8749(90)90034-o. [DOI] [PubMed] [Google Scholar]
- Schäfer H., Müller B., Bader A., Schenkel J., Burger R. Analysis of guinea pig leukocyte antigens using interspecies T cell hybrids. J Immunol Methods. 1989 Mar 31;118(2):169–177. doi: 10.1016/0022-1759(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Dialynas D. P., Fitch F. W., English M. Monoclonal antibody to L3T4 blocks the function of T cells specific for class 2 major histocompatibility complex antigens. J Immunol. 1984 Mar;132(3):1118–1123. [PubMed] [Google Scholar]
- Tourvieille B., Gorman S. D., Field E. H., Hunkapiller T., Parnes J. R. Isolation and sequence of L3T4 complementary DNA clones: expression in T cells and brain. Science. 1986 Oct 31;234(4776):610–614. doi: 10.1126/science.3094146. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Wassmer P., Chan C., Lögdberg L., Shevach E. M. Role of the L3T4-antigen in T cell activation. II. Inhibition of T cell activation by monoclonal anti-L3T4 antibodies in the absence of accessory cells. J Immunol. 1985 Oct;135(4):2237–2242. [PubMed] [Google Scholar]
- Webb M., Mason D. W., Williams A. F. Inhibition of mixed lymphocyte response by monoclonal antibody specific for a rat T lymphocyte subset. Nature. 1979 Dec 20;282(5741):841–843. doi: 10.1038/282841a0. [DOI] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]