Abstract
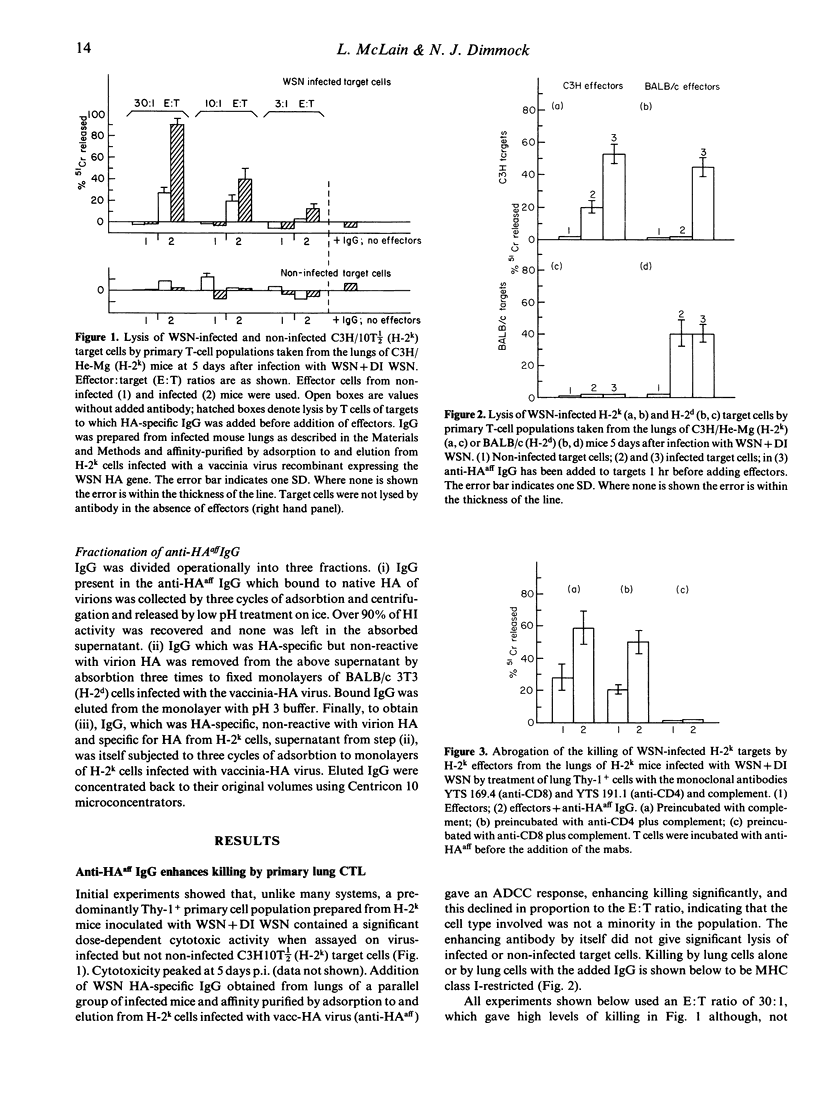
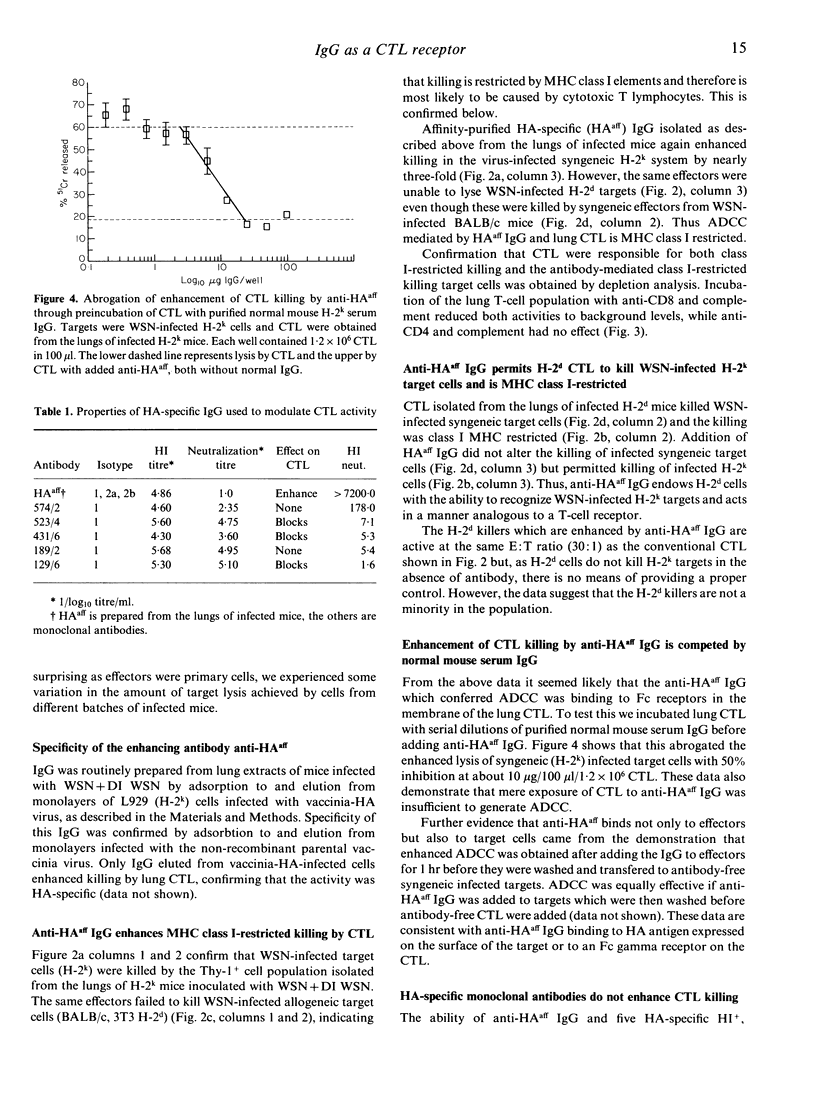
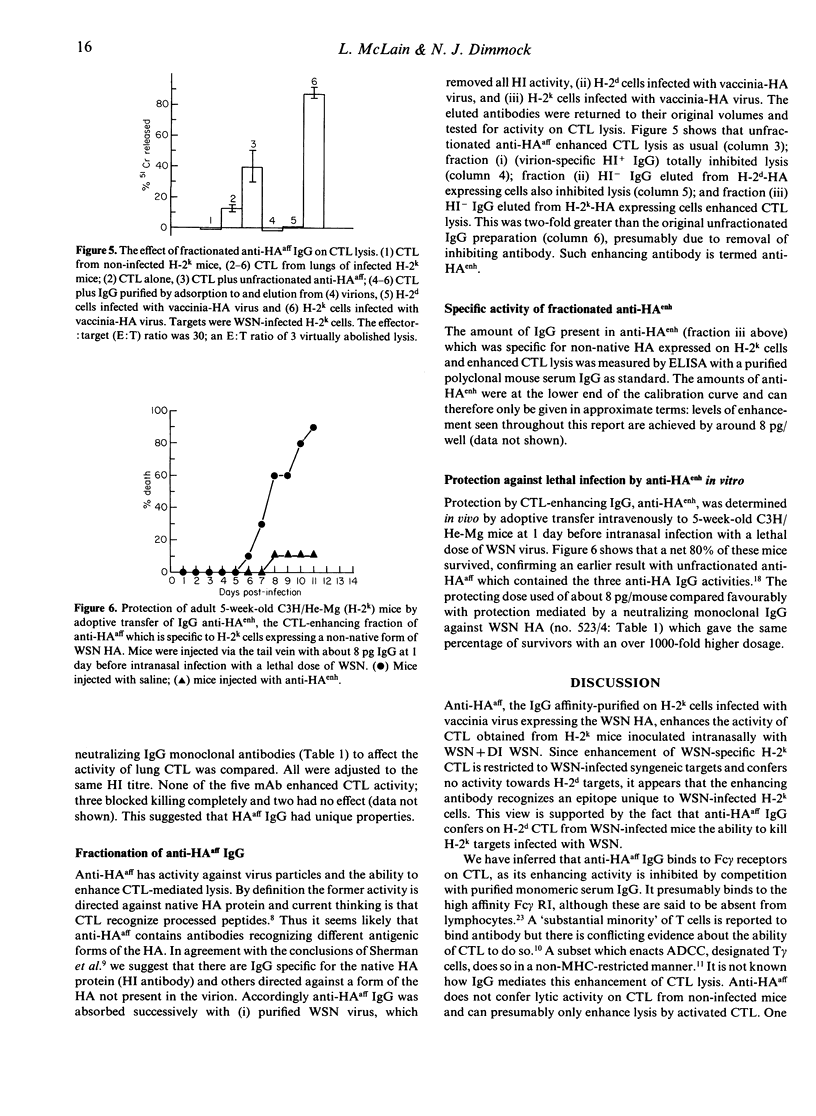
Lungs of H-2k mice co-inoculated with type A/WSN influenza virus+detective interfering WSN virus contain haemagglutinin (HA)-specific IgG which have three different activities. These have been purified by adsorbtion and elution using different forms of HA. The first IgG recognizes HA in a form present on H-2k cells infected with a vaccinia virus recombinant expressing the WSN HA gene (vaccinia-HA virus), but not on virus particles, and enhances class I major histocompatibility complex (MHC)-restricted killing of WSN-infected H-2k target cells by primary cytotoxic T lymphocytes (CTL) from the lungs of WSN-infected H-2k mice; it also confers on primary CTL from the lungs of WSN-infected H-2d mice the ability to lyse WSN-infected H-2k targets. This IgG is therefore analogous to the T-cell receptor in that it is antigen specific and MHC restricted. A second IgG recognizes HA in a form present on both H-2k and H-2d cells infected with the vaccinia-HA virus but not present on virus particles and inhibits CTL lysis of WSN-infected syngeneic target cells. Only the third binds to virus particles; this inhibits agglutination of red cells, but is non-neutralizing. It also inhibits CTL lysis of WSN-infected syngeneic targets. Thus we present evidence that HA-specific IgG may have a significant role in regulating CTL responses to influenza virus in vivo and that one of these IgG is MHC-restricted in its recognition of viral antigen. Finally, in vivo significance of these antibodies is indicated by the finding that adoptively transferred CTL-enhancing IgG protects mice from lethal WSN infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett B. C., Graham C. M., Burt D. S., Skehel J. J., Thomas D. B. The immune response of BALB/c mice to influenza hemagglutinin: commonality of the B cell and T cell repertoires and their relevance to antigenic drift. Eur J Immunol. 1989 Mar;19(3):515–521. doi: 10.1002/eji.1830190316. [DOI] [PubMed] [Google Scholar]
- Barrett A. D., Dimmock N. J. Defective interfering viruses and infections of animals. Curr Top Microbiol Immunol. 1986;128:55–84. doi: 10.1007/978-3-642-71272-2_2. [DOI] [PubMed] [Google Scholar]
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Dickler H. B. Lymphocyte receptors for immunoglobulin. Adv Immunol. 1976;24:167–214. doi: 10.1016/s0065-2776(08)60330-2. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J., Beck S., McLain L. Protection of mice from lethal influenza: evidence that defective interfering virus modulates the immune response and not virus multiplication. J Gen Virol. 1986 May;67(Pt 5):839–850. doi: 10.1099/0022-1317-67-5-839. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Effros R. B., Frankel M. E., Gerhard W., Doherty P. C. Inhibition of influenza-immune T cell effector function by virus-specific hybridoma antibody. J Immunol. 1979 Sep;123(3):1343–1346. [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Burakoff S. J., Fields B. N. Type-specific reovirus antiserum blocks the cytotoxic T-cell-target cell interaction: evidence for the association of the viral hemagglutinin of a nonenveloped virus with the cell surface. Infect Immun. 1981 Feb;31(2):646–649. doi: 10.1128/iai.31.2.646-649.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C. M., Barnett B. C., Hartlmayr I., Burt D. S., Faulkes R., Skehel J. J., Thomas D. B. The structural requirements for class II (I-Ad)-restricted T cell recognition of influenza hemagglutinin: B cell epitopes define T cell epitopes. Eur J Immunol. 1989 Mar;19(3):523–528. doi: 10.1002/eji.1830190317. [DOI] [PubMed] [Google Scholar]
- Hale A. H., Witte O. N., Baltimore D., Eisen H. N. Vesicular stomatitis virus glycoprotein is necessary for H-2-restricted lysis of infected cells by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):970–974. doi: 10.1073/pnas.75.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Thomssen R. Target cell-dependent T cell-mediated lysis of vaccinia virus-infected cells. Eur J Immunol. 1975 Apr;5(4):245–251. doi: 10.1002/eji.1830050405. [DOI] [PubMed] [Google Scholar]
- McLain L., Armstrong S. J., Dimmock N. J. One defective interfering particle per cell prevents influenza virus-mediated cytopathology: an efficient assay system. J Gen Virol. 1988 Jun;69(Pt 6):1415–1419. doi: 10.1099/0022-1317-69-6-1415. [DOI] [PubMed] [Google Scholar]
- McLain L., Dimmock N. J. Protection of mice from lethal influenza by adoptive transfer of non-neutralizing haemagglutination-inhibiting IgG obtained from the lungs of infected animals treated with defective interfering virus. J Gen Virol. 1989 Oct;70(Pt 10):2615–2624. doi: 10.1099/0022-1317-70-10-2615. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Gell P. G. Cell-mediated immunity in herpes simplex virus-infected mice: suppression of delayed hypersensitivity by an antigen-specific B lymphocyte. J Gen Virol. 1980 Jun;48(Pt 2):359–364. doi: 10.1099/0022-1317-48-2-359. [DOI] [PubMed] [Google Scholar]
- Pang T., Blanden R. V. Regulation of the T-cell response to ectromelia virus infection. I. Feedback suppression by effector T cells. J Exp Med. 1976 Mar 1;143(3):469–481. doi: 10.1084/jem.143.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J. Origin and replication of defective interfering particles. Curr Top Microbiol Immunol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- Senik A., Neauport-Sautes C. Association between H-2 and vaccinia virus-induced antigens on the surface of infected cells. J Immunol. 1979 Apr;122(4):1461–1467. [PubMed] [Google Scholar]
- Sherman L. A., Vitiello A., Klinman N. R. T-cell and B-cell responses to viral antigens at the clonal level. Annu Rev Immunol. 1983;1:63–86. doi: 10.1146/annurev.iy.01.040183.000431. [DOI] [PubMed] [Google Scholar]
- Spits H., van Schooten W., Keizer H., van Seventer G., van de Rijn M., Terhorst C., de Vries J. E. Alloantigen recognition is preceded by nonspecific adhesion of cytotoxic T cells and target cells. Science. 1986 Apr 18;232(4748):403–405. doi: 10.1126/science.3485822. [DOI] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W., Earl P., Moss B. Surface expression of viral glycoproteins is polarized in epithelial cells infected with recombinant vaccinia viral vectors. EMBO J. 1986 Feb;5(2):237–245. doi: 10.1002/j.1460-2075.1986.tb04204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser M. T., Braciale V. L., Braciale T. J. Class I major histocompatibility complex-restricted T lymphocyte recognition of the influenza hemagglutinin. Overlap between class I cytotoxic T lymphocytes and antibody sites. J Exp Med. 1989 Oct 1;170(4):1357–1368. doi: 10.1084/jem.170.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen W. L., Wraith D., Barber B. H. Searching for MHC-restricted anti-viral antibodies: antibodies recognizing the nucleoprotein of influenza virus dominate the serological response of C57BL/6 mice to syngeneic influenza-infected cells. Eur J Immunol. 1987 Jul;17(7):999–1006. doi: 10.1002/eji.1830170716. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Scigliano E., Freedman V. H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- Voss E. W., Jr Anti-metatype antibody reactivity: a model for T-cell receptor recognition. Immunol Today. 1990 Oct;11(10):355–357. doi: 10.1016/0167-5699(90)90140-5. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]


