Abstract
The presence of androgen receptors in thymocytes and the well-described effects of exogenous androgens on thymus size suggest a role for androgenic hormones in thymocyte growth and maturation. Testicular feminization (Tfm/Y) mice which bear a heritable defect in the androgen receptor protein were studied to investigate how androgens might influence immune phenotype and function. These mice were compared to two types of controls; their Tabby/Y normal male littermates and male mice of the C57 Bl/6 strain from which the Tabby and Tfm mice were derived. Thymuses and spleens from Tfm/Y mice were larger than both types of controls. Phenotypic differences in thymocyte and splenocyte subpopulations identified by the T-cell markers CD3, CD4 and CD8 suggested that T-cell maturation was altered in the androgen-resistant animal. However, both Ta/Y and Tfm/Y were found to be high producers of interleukin-4 (IL-4) by both spleen and thymus cells, while cells from the C57 mice produced predominantly IL-2. These findings suggest that some immunological features of the Tfm/Y mouse may be related to its defect in androgen action, but that high levels of IL-4 production are probably related to other genetic changes in the C57 background.
Full text
PDF

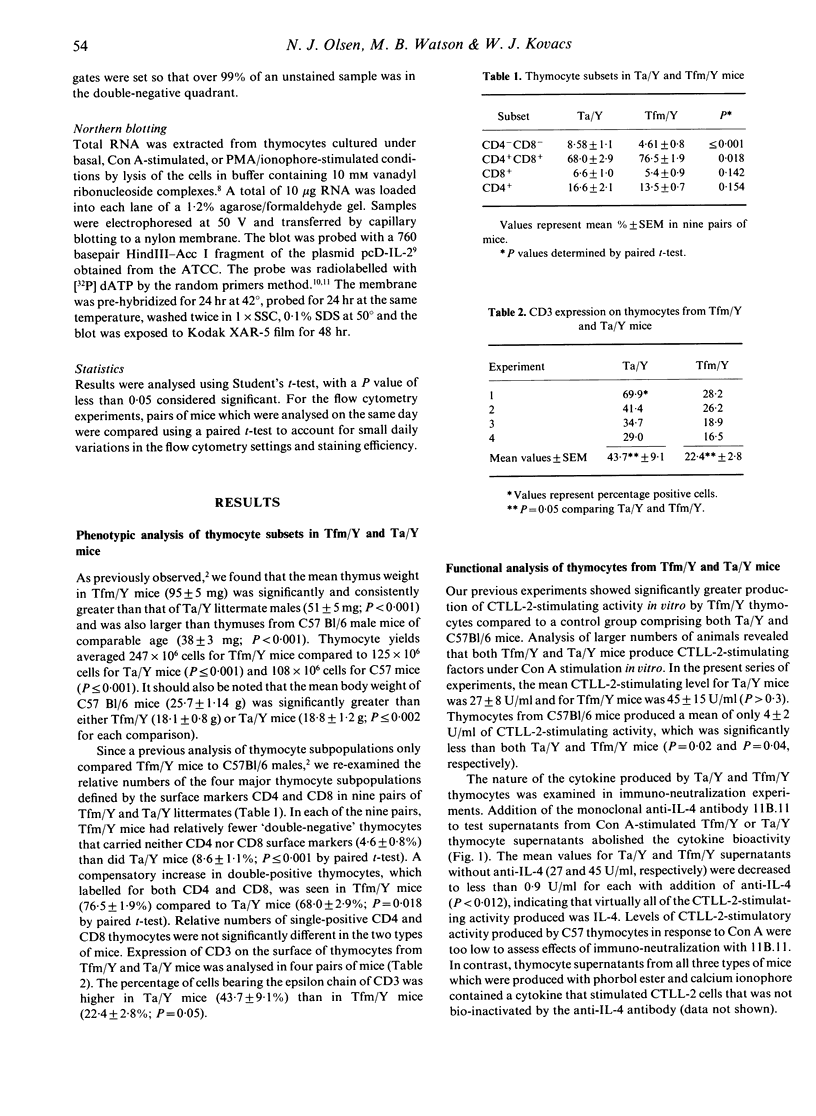
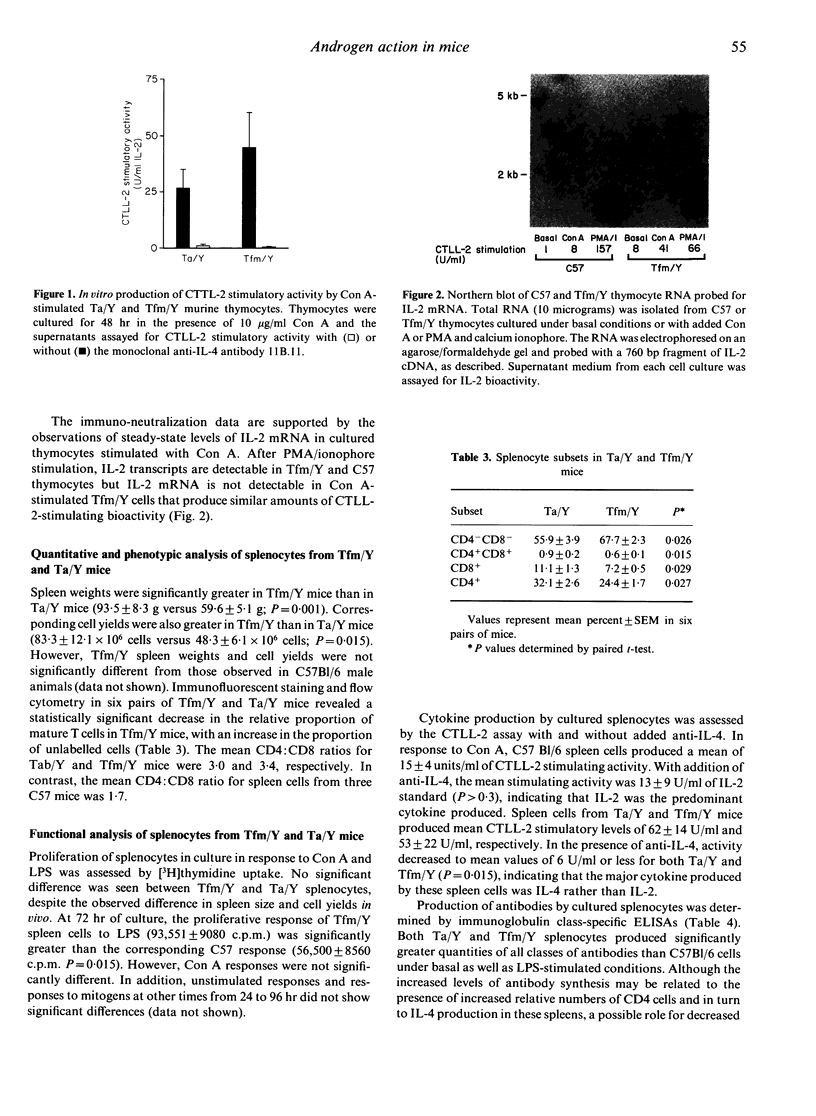


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Grabstein K., Eisenman J., Mochizuki D., Shanebeck K., Conlon P., Hopp T., March C., Gillis S. Purification to homogeneity of B cell stimulating factor. A molecule that stimulates proliferation of multiple lymphokine-dependent cell lines. J Exp Med. 1986 Jun 1;163(6):1405–1414. doi: 10.1084/jem.163.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs W. J., Olsen N. J. Androgen receptors in human thymocytes. J Immunol. 1987 Jul 15;139(2):490–493. [PubMed] [Google Scholar]
- Lewis D. B., Prickett K. S., Larsen A., Grabstein K., Weaver M., Wilson C. B. Restricted production of interleukin 4 by activated human T cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9743–9747. doi: 10.1073/pnas.85.24.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Olsen N. J., Kovacs W. J. Increased thymic size and thymocyte interleukin 2 production in androgen-resistant mice. Scand J Immunol. 1989 Jun;29(6):733–738. doi: 10.1111/j.1365-3083.1989.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Pearse M., Gallagher P., Wilson A., Wu L., Fisicaro N., Miller J. F., Scollay R., Shortman K. Molecular characterization of T-cell antigen receptor expression by subsets of CD4- CD8- murine thymocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6082–6086. doi: 10.1073/pnas.85.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Ransom J., Fischer M., Mosmann T., Yokota T., DeLuca D., Schumacher J., Zlotnik A. Interferon-gamma is produced by activated immature mouse thymocytes and inhibits the interleukin 4-induced proliferation of immature thymocytes. J Immunol. 1987 Dec 15;139(12):4102–4108. [PubMed] [Google Scholar]
- Sideras P., Funa K., Zalcberg-Quintana I., Xanthopoulos K. G., Kisielow P., Palacios R. Analysis by in situ hybridization of cells expressing mRNA for interleukin 4 in the developing thymus and in peripheral lymphocytes from mice. Proc Natl Acad Sci U S A. 1988 Jan;85(1):218–221. doi: 10.1073/pnas.85.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Ransom J., Frank G., Fischer M., Howard M. Interleukin 4 is a growth factor for activated thymocytes: possible role in T-cell ontogeny. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3856–3860. doi: 10.1073/pnas.84.11.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]



