Abstract
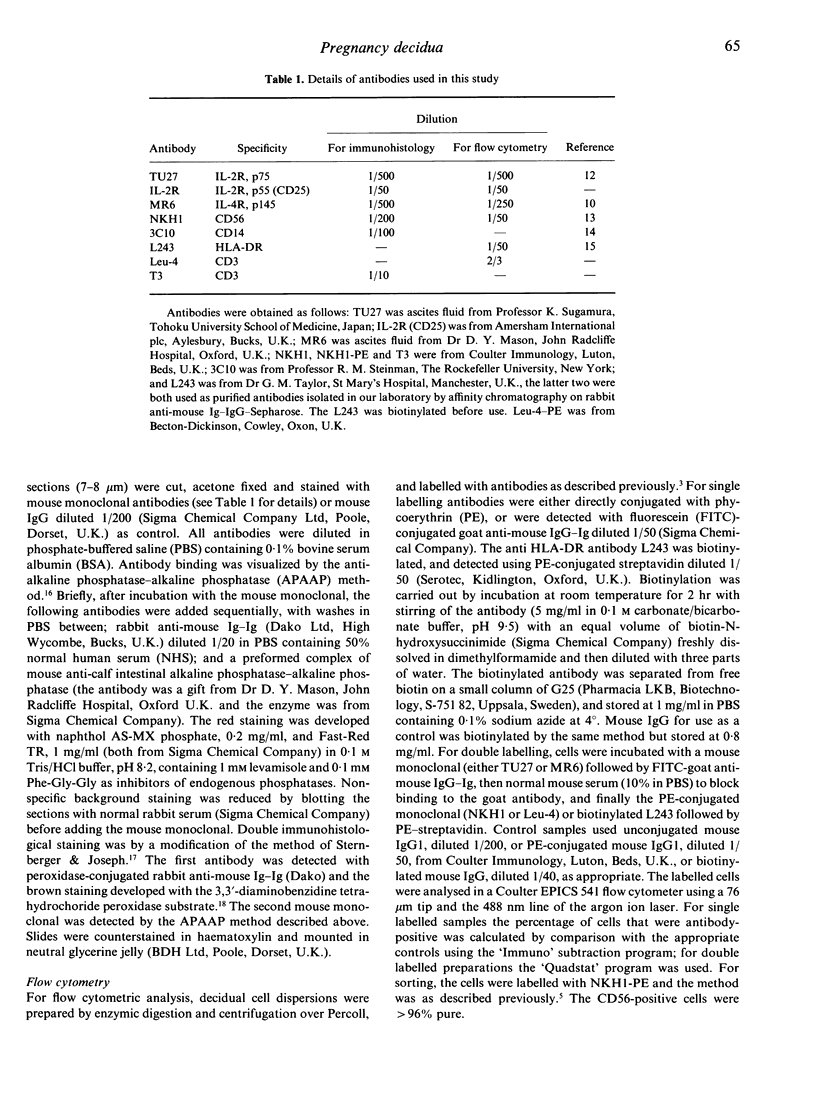
Immunohistological studies of human first trimester pregnancy decidua demonstrated the presence of the p75 interleukin-2 receptor (IL-2R) and the p145 interleukin-4 receptor protein (IL-4R) on cells in the decidual stroma; there was no expression of CD25, the p55 IL-2R. The IL-4R was also expressed on the basal face of the glandular epithelial cells. Flow cytometric analysis of antibody-labelled decidual cell dispersions confirmed these results. Double antibody labelling demonstrated that p75 was expressed exclusively on the CD56-positive decidual large granular lymphocytes (LGL), whereas the IL4-R was expressed on some decidual LGL, and most decidual macrophages and T cells. In vitro incubation of decidual cells with IL-2 failed to induce expression of p55 or to increase the expression of either p75 or the p145 IL-4R. Purified decidual LGL proliferated in vitro in response to IL-2, and IL-4 inhibited this IL-2-induced proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al Jabaari B., Ladyman H. M., Larché M., Sivolapenko G. B., Epenetos A. A., Ritter M. A. Elevated expression of the interleukin 4 receptor in carcinoma: a target for immunotherapy? Br J Cancer. 1989 Jun;59(6):910–914. doi: 10.1038/bjc.1989.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer J. N., Johnson P. M. Immunohistological characterization of the decidual leucocytic infiltrate related to endometrial gland epithelium in early human pregnancy. Immunology. 1985 May;55(1):35–44. [PMC free article] [PubMed] [Google Scholar]
- Bulmer J. N., Johnson P. M. The T-lymphocyte population in first-trimester human decidua does not express the interleukin-2 receptor. Immunology. 1986 Aug;58(4):685–687. [PMC free article] [PubMed] [Google Scholar]
- Bulmer J. N., Sunderland C. A. Immunohistological characterization of lymphoid cell populations in the early human placental bed. Immunology. 1984 Jun;52(2):349–357. [PMC free article] [PubMed] [Google Scholar]
- Chapple M. R., Johnson G. D., Davidson R. S. Fluorescence quenching of fluorescein by R-phycoerythrin. A pitfall in dual fluorescence analysis. J Immunol Methods. 1988 Jul 22;111(2):209–218. doi: 10.1016/0022-1759(88)90129-9. [DOI] [PubMed] [Google Scholar]
- Clark D. A., Slapsys R., Chaput A., Walker C., Brierley J., Daya S., Rosenthal K. L. Immunoregulatory molecules of trophoblast and decidual suppressor cell origin at the maternofetal interface. Am J Reprod Immunol Microbiol. 1986 Mar;10(3):100–104. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Donnelly R. P., Fenton M. J., Finbloom D. S., Gerrard T. L. Differential regulation of IL-1 production in human monocytes by IFN-gamma and IL-4. J Immunol. 1990 Jul 15;145(2):569–575. [PubMed] [Google Scholar]
- Ellis T. M., Fisher R. I. Functional heterogeneity of Leu 19"bright"+ and Leu 19"dim"+ lymphokine-activated killer cells. J Immunol. 1989 Apr 15;142(8):2949–2954. [PubMed] [Google Scholar]
- Ferry B. L., Sargent I. L., Starkey P. M., Redman C. W. Cytotoxic activity against trophoblast and choriocarcinoma cells of large granular lymphocytes from human early pregnancy decidua. Cell Immunol. 1991 Jan;132(1):140–149. doi: 10.1016/0008-8749(91)90013-2. [DOI] [PubMed] [Google Scholar]
- Ferry B. L., Starkey P. M., Sargent I. L., Watt G. M., Jackson M., Redman C. W. Cell populations in the human early pregnancy decidua: natural killer activity and response to interleukin-2 of CD56-positive large granular lymphocytes. Immunology. 1990 Aug;70(4):446–452. [PMC free article] [PubMed] [Google Scholar]
- Gerrard T. L., Dyer D. R., Mostowski H. S. IL-4 and granulocyte-macrophage colony-stimulating factor selectively increase HLA-DR and HLA-DP antigens but not HLA-DQ antigens on human monocytes. J Immunol. 1990 Jun 15;144(12):4670–4674. [PubMed] [Google Scholar]
- Griffin J. D., Hercend T., Beveridge R., Schlossman S. F. Characterization of an antigen expressed by human natural killer cells. J Immunol. 1983 Jun;130(6):2947–2951. [PubMed] [Google Scholar]
- Idzerda R. L., March C. J., Mosley B., Lyman S. D., Vanden Bos T., Gimpel S. D., Din W. S., Grabstein K. H., Widmer M. B., Park L. S. Human interleukin 4 receptor confers biological responsiveness and defines a novel receptor superfamily. J Exp Med. 1990 Mar 1;171(3):861–873. doi: 10.1084/jem.171.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Larche M., Lamb J. R., O'Hehir R. E., Imami-Shita N., Zanders E. D., Quint D. E., Moqbel R., Ritter M. A. Functional evidence for a monoclonal antibody that binds to the human IL-4 receptor. Immunology. 1988 Dec;65(4):617–622. [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Gnarra J. R., Napolitano M., Sharon M. Structure, function, and regulation of the interleukin-2 receptor and identification of a novel immune activation gene. Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):187–192. doi: 10.1098/rstb.1990.0053. [DOI] [PubMed] [Google Scholar]
- Lewis C. E., McCarthy S. P., Richards P. S., Lorenzen J., Horak E., McGee J. O. Measurement of cytokine release by human cells. A quantitative analysis at the single cell level using the reverse haemolytic plaque assay. J Immunol Methods. 1990 Feb 20;127(1):51–59. doi: 10.1016/0022-1759(90)90340-2. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Castle B. E., Christiansen J., Schreurs J., Rennick D., Arai N., Hoy P., Takebe Y., Howard M. Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J Immunol. 1988 Jan 15;140(2):456–464. [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S., Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–3191. [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Phillips J. H. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990 May 1;171(5):1527–1533. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Sassenfeld H. M., Urdal D. L. Characterization of the human B cell stimulatory factor 1 receptor. J Exp Med. 1987 Aug 1;166(2):476–488. doi: 10.1084/jem.166.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Takeshita T., Sugamura K., Lanier L. L. Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Jul 1;170(1):291–296. doi: 10.1084/jem.170.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Tanaka Y., Eto S., Suzuki H., Yodoi J., Yamashita U. Effect of interleukin 1 on the expression of interleukin 2 receptor (Tac antigen) on human natural killer cells and natural killer-like cell line (YT cells). J Immunol. 1986 Jul 15;137(2):551–556. [PubMed] [Google Scholar]
- Spits H., Yssel H., Paliard X., Kastelein R., Figdor C., de Vries J. E. IL-4 inhibits IL-2-mediated induction of human lymphokine-activated killer cells, but not the generation of antigen-specific cytotoxic T lymphocytes in mixed leukocyte cultures. J Immunol. 1988 Jul 1;141(1):29–36. [PubMed] [Google Scholar]
- Starkey P. M. Reactivity of human trophoblast with an antibody to the HLA class II antigen, HLA-DP. J Reprod Immunol. 1987 May;11(1):63–70. doi: 10.1016/0165-0378(87)90007-6. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Sargent I. L., Redman C. W. Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology. 1988 Sep;65(1):129–134. [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Joseph S. A. The unlabeled antibody method. Contrasting color staining of paired pituitary hormones without antibody removal. J Histochem Cytochem. 1979 Nov;27(11):1424–1429. doi: 10.1177/27.11.92498. [DOI] [PubMed] [Google Scholar]
- Sutton L., Gadd M., Mason D. Y., Redman C. W. Cells bearing class II MHC antigens in the human placenta and amniochorion. Immunology. 1986 May;58(1):23–29. [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann T. G. Placental immunotrophism: maternal T cells enhance placental growth and function. Am J Reprod Immunol Microbiol. 1987 Oct;15(2):67–69. doi: 10.1111/j.1600-0897.1987.tb00156.x. [DOI] [PubMed] [Google Scholar]



