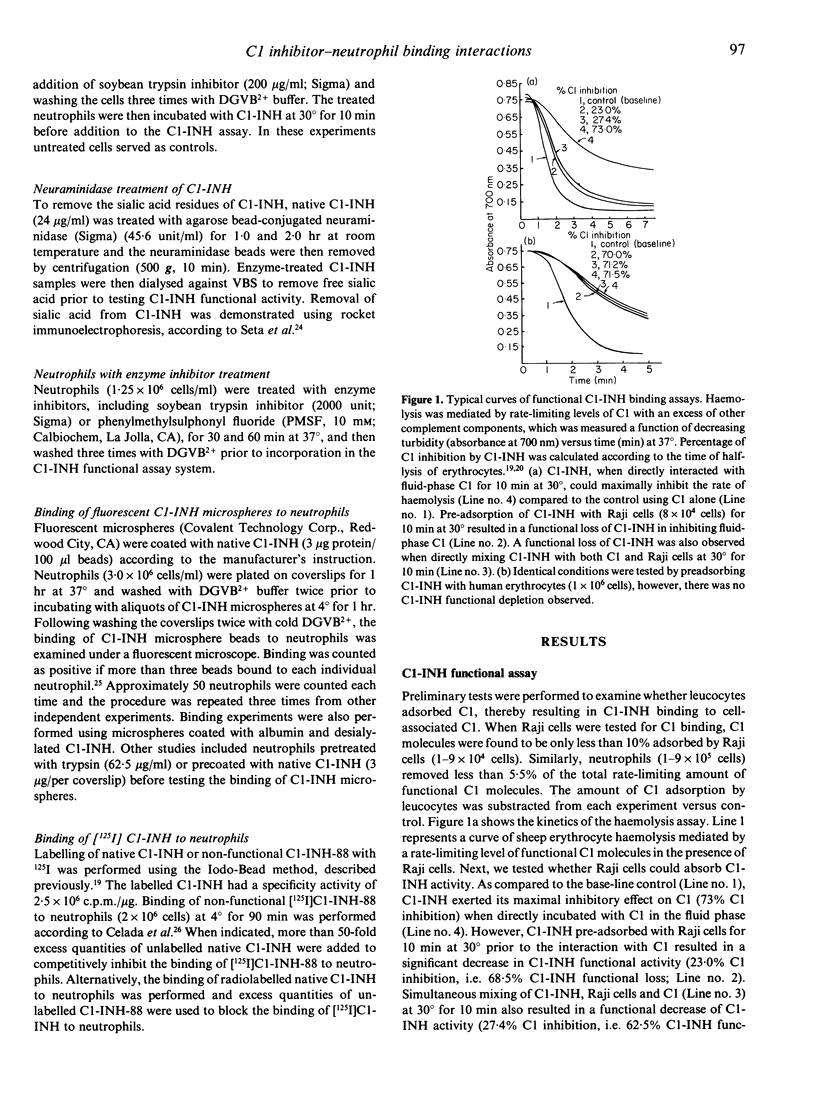
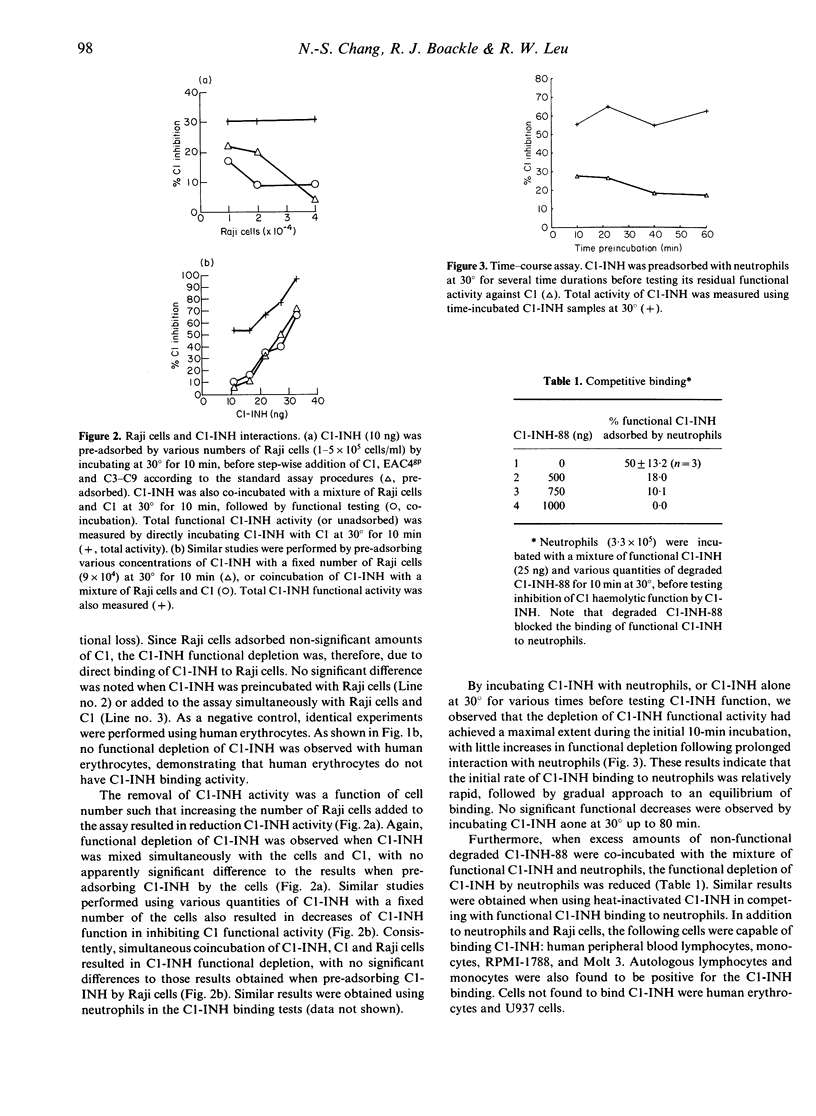
Abstract
In a previous study we have isolated neutrophil membrane proteins that non-covalently bind to native C1-INH (105,000 MW) and a non-functional, degraded C1-INH (88,000 MW; C1-INH-88). To further characterize the binding nature, we have designed a novel kinetic C1 titration assay which enables not only a quantification of the removal of fluid-phase C1-INH by neutrophils, but also a concomitant measure of residual C1-INH function. Native C1-INH, when adsorbed to EDTA-pretreated neutrophils, lost its function in the inhibition of fluid-phase C1. The non-functional C1-INH-88, which is probably devoid of a reactive centre, was found to block the binding of native C1-INH to neutrophils. Pretreatment of neutrophils with serine esterase inhibitors did not abrogate binding capacity of the cells for C1-INH, whereas the binding affinity for C1-INH was lost when the cells were pretreated with trypsin. An array of human peripheral blood leucocytes and several lymphoid cell lines has surface binding sites for C1-INH, but not on human erythrocytes and U937 cells. Binding was further confirmed using (i) C1-INH-microsphere beads to neutrophils, in which the binding was blocked when pretreating neutrophils with excess C1-INH or with trypsin, and (ii) radiolabelled C1-INH to neutrophils, which was competitively blocked by unlabelled non-functional C1-INH-88. Desialylation of C1-INH significantly reduced its binding affinity for neutrophils, indicating that the membrane receptor sites on neutrophils could be specific for the binding of sialic acid residues on C1-INH. Overall, our studies indicate that neutrophils or other leucocytes possess specific surface binding sites for the sialic acid-containing portion of C1-INH.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach-Martensen N., Osther K., Stroyer I. Letter: C1-esterase inactivators and C4 in malignant diseases. Lancet. 1975 Sep 13;2(7933):499–500. doi: 10.1016/s0140-6736(75)90571-1. [DOI] [PubMed] [Google Scholar]
- Boackle R. J., Pruitt K. M., Mestecky J. The interactions of human complement with interfacially aggregated preparations of human secretory IgA. Immunochemistry. 1974 Sep;11(9):543–548. doi: 10.1016/0019-2791(74)90245-6. [DOI] [PubMed] [Google Scholar]
- Bock S. C., Skriver K., Nielsen E., Thøgersen H. C., Wiman B., Donaldson V. H., Eddy R. L., Marrinan J., Radziejewska E., Huber R. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry. 1986 Jul 29;25(15):4292–4301. doi: 10.1021/bi00363a018. [DOI] [PubMed] [Google Scholar]
- Boltz-Nitulescu G., Ortel B., Riedl M., Förster O. Ganglioside receptor of rat macrophages. Modulation by enzyme treatment and evidence for its protein nature. Immunology. 1984 Jan;51(1):177–184. [PMC free article] [PubMed] [Google Scholar]
- Caughman G. B., Boackle R. J., Vesely J. A postulated mechanism for heparin's potentiation of C1 inhibitor function. Mol Immunol. 1982 Feb;19(2):287–295. doi: 10.1016/0161-5890(82)90342-x. [DOI] [PubMed] [Google Scholar]
- Celada A., Allen R., Esparza I., Gray P. W., Schreiber R. D. Demonstration and partial characterization of the interferon-gamma receptor on human mononuclear phagocytes. J Clin Invest. 1985 Dec;76(6):2196–2205. doi: 10.1172/JCI112228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil B. J., Chaitovitz S., Wong C., Pillai S. Molecular cloning of a human macrophage lectin specific for galactose. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7324–7328. doi: 10.1073/pnas.87.18.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R. Activation and regulation of the first complement component. Fed Proc. 1983 Jan;42(1):134–138. [PubMed] [Google Scholar]
- Davis A. E., 3rd C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol. 1988;6:595–628. doi: 10.1146/annurev.iy.06.040188.003115. [DOI] [PubMed] [Google Scholar]
- Forbes C. D., Pensky J., Ratnoff O. D. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J Lab Clin Med. 1970 Nov;76(5):809–815. [PubMed] [Google Scholar]
- Funk W. G., Lasser E. C., Lang J. H., Intaglietta M., Hamblin A. E. Effect of elevated C1-esterase inhibitor concentrations on white blood cell-endothelium interactions: a potential mechanism for steroid protection in contrast material reactions. Invest Radiol. 1982 Mar-Apr;17(2):189–192. doi: 10.1097/00004424-198203000-00016. [DOI] [PubMed] [Google Scholar]
- Gessl A., Boltz-Nitulescu G., Wiltschke C., Holzinger C., Nemet H., Pernerstorfer T., Förster O. Expression of a binding structure for sialic acid-containing glycoconjugates on rat bone marrow-derived macrophages and its modulation by IFN, TNF-alpha, and dexamethasone. J Immunol. 1989 Jun 15;142(12):4372–4377. [PubMed] [Google Scholar]
- Harrison R. A. Human C1 inhibitor: improved isolation and preliminary structural characterization. Biochemistry. 1983 Oct 11;22(21):5001–5007. doi: 10.1021/bi00290a019. [DOI] [PubMed] [Google Scholar]
- Haupt H., Heimburger N., Kranz T., Schwick H. G. Ein Beitrag zur Isolierung und Charakterisierung des Cl-Inaktivators aus Humanplasma. Eur J Biochem. 1970 Dec;17(2):254–261. doi: 10.1111/j.1432-1033.1970.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Ii M., Kurata H., Itoh N., Yamashina I., Kawasaki T. Molecular cloning and sequence analysis of cDNA encoding the macrophage lectin specific for galactose and N-acetylgalactosamine. J Biol Chem. 1990 Jul 5;265(19):11295–11298. [PubMed] [Google Scholar]
- Kitamura H., Teshima H., Day N. K. The presence of active C1 (C-1) on peripheral human lymphocytes. Vox Sang. 1978;35(4):197–206. doi: 10.1111/j.1423-0410.1978.tb02922.x. [DOI] [PubMed] [Google Scholar]
- Minta J. O. The role of sialic acid in the functional activity and the hepatic clearance of C1-INH. J Immunol. 1981 Jan;126(1):245–249. [PubMed] [Google Scholar]
- Mirro J., Jr, Schwartz J. F., Civin C. I. Simultaneous analysis of cell surface antigens and cell morphology using monoclonal antibodies conjugated to fluorescent microspheres. J Immunol Methods. 1981;47(1):39–48. doi: 10.1016/0022-1759(81)90255-6. [DOI] [PubMed] [Google Scholar]
- Osther K., Hojgaard K., Dybkjaer E. Demonstration of a complement of inactivator on cultured cells from human malignant brain tumours. Acta Neurol Scand. 1974;50(6):681–689. doi: 10.1111/j.1600-0404.1974.tb02814.x. [DOI] [PubMed] [Google Scholar]
- Osther K., Linnemann R. Immunofluorescence measurement of C1 inactivator (alpha 2 neuraminoglycoprotein) activity of the surface of human carcinoma cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Jun;81(3):365–372. doi: 10.1111/j.1699-0463.1973.tb02217.x. [DOI] [PubMed] [Google Scholar]
- Osther K., Linnemann R. Measurement of C1 inactivator (alpha 2 neuraminoglycoprotein) on human blast cells in blast leukaemia. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Apr;81(2):271–272. doi: 10.1111/j.1699-0463.1973.tb00224.x. [DOI] [PubMed] [Google Scholar]
- PENSKY J., LEVY L. R., LEPOW I. H. Partial purification of a serum inhibitor of C'1-esterase. J Biol Chem. 1961 Jun;236:1674–1679. [PubMed] [Google Scholar]
- Patrick R. A., Hollers J. C., Liu D. Y., Giese B. H., Smith C. W. Effects of human complement component 1 inactivator on neutrophil chemotaxis and chemotactic deactivation. Infect Immun. 1980 Jun;28(3):700–707. doi: 10.1128/iai.28.3.700-707.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo B. P., Fleit H. B., Kaplan A. P., Ghebrehiwet B. Expression of functional cell surface C1-inactivator by U937 cells. Clin Immunol Immunopathol. 1988 Dec;49(3):463–477. doi: 10.1016/0090-1229(88)90133-x. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D., Pensky J., Ogston D., Naff G. B. The inhibition of plasmin, plasma kallikrein, plasma permeability factor, and the C'1r subcomponent of the first component of complement by serum C'1 esterase inhibitor. J Exp Med. 1969 Feb 1;129(2):315–331. doi: 10.1084/jem.129.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul A., Arlaud G. J., Sim R. B., Colomb M. G. A simplified procedure for the purification of C1-inactivator from human plasma. Interaction with complement subcomponents C1r and C1s. FEBS Lett. 1977 Jul 1;79(1):45–50. doi: 10.1016/0014-5793(77)80347-5. [DOI] [PubMed] [Google Scholar]
- Remold H. G., Rosenberg R. D. Enhancement of migration inhibitory factor activity by plasma esterase inhibitors. J Biol Chem. 1975 Aug 25;250(16):6608–6613. [PubMed] [Google Scholar]
- Schreiber A. D., Kaplan A. P., Austen K. F. Plasma inhibitors of the components of the fibrinolytic pathway in man. J Clin Invest. 1973 Jun;52(6):1394–1401. doi: 10.1172/JCI107312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbource-Goguel Seta N. S., Bordas M. C., Féger J. M., Davy J. A., Durand G. M. Evaluation of the degree of desialylation of serum C1-inactivator and haemopexin. Clin Chim Acta. 1984 Nov 30;143(3):235–241. doi: 10.1016/0009-8981(84)90073-1. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Bing D. H., Patrick R. A. Effects of human C 1 inhibitor on complement-mediated human leukocyte chemotaxis. J Immunol. 1975 Jan;114(1 Pt 1):216–220. [PubMed] [Google Scholar]


